Phosphatidylinositol 3-kinase class II α-isoform PI3K-C2α is required for transforming growth factor β-induced Smad signaling in endothelial cells
- PMID: 25614622
- PMCID: PMC4358250
- DOI: 10.1074/jbc.M114.601484
Phosphatidylinositol 3-kinase class II α-isoform PI3K-C2α is required for transforming growth factor β-induced Smad signaling in endothelial cells
Abstract
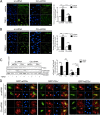
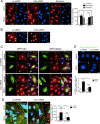
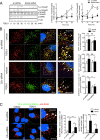
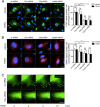
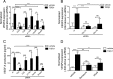
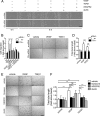
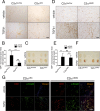
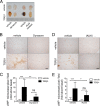
We have recently demonstrated that the PI3K class II-α isoform (PI3K-C2α), which generates phosphatidylinositol 3-phosphate and phosphatidylinositol 3,4-bisphosphates, plays crucial roles in angiogenesis, by analyzing PI3K-C2α knock-out mice. The PI3K-C2α actions are mediated at least in part through its participation in the internalization of VEGF receptor-2 and sphingosine-1-phosphate receptor S1P1 and thereby their signaling on endosomes. TGFβ, which is also an essential angiogenic factor, signals via the serine/threonine kinase receptor complex to induce phosphorylation of Smad2 and Smad3 (Smad2/3). SARA (Smad anchor for receptor activation) protein, which is localized in early endosomes through its FYVE domain, is required for Smad2/3 signaling. In the present study, we showed that PI3K-C2α knockdown nearly completely abolished TGFβ1-induced phosphorylation and nuclear translocation of Smad2/3 in vascular endothelial cells (ECs). PI3K-C2α was necessary for TGFβ-induced increase in phosphatidylinositol 3,4-bisphosphates in the plasma membrane and TGFβ receptor internalization into the SARA-containing early endosomes, but not for phosphatidylinositol 3-phosphate enrichment or localization of SARA in the early endosomes. PI3K-C2α was also required for TGFβ receptor-mediated formation of SARA-Smad2/3 complex. Inhibition of dynamin, which is required for the clathrin-dependent receptor endocytosis, suppressed both TGFβ receptor internalization and Smad2/3 phosphorylation. TGFβ1 stimulated Smad-dependent VEGF-A expression, VEGF receptor-mediated EC migration, and capillary-like tube formation, which were all abolished by either PI3K-C2α knockdown or a dynamin inhibitor. Finally, TGFβ1-induced microvessel formation in Matrigel plugs was greatly attenuated in EC-specific PI3K-C2α-deleted mice. These observations indicate that PI3K-C2α plays the pivotal role in TGFβ receptor endocytosis and thereby Smad2/3 signaling, participating in angiogenic actions of TGFβ.
Keywords: Endosome; Endothelial Cell; PI3K-C2alpha; Phosphatidylinositol Kinase (PI Kinase); Receptor Endocytosis; SARA; SMAD Transcription Factor; TGF-B Receptor; Transforming Growth Factor Beta (TGF-B); Vascular Endothelial Growth Factor (VEGF).
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




Similar articles
-
Class II phosphatidylinositol 3-kinase-C2α is essential for Notch signaling by regulating the endocytosis of γ-secretase in endothelial cells.Sci Rep. 2021 Mar 4;11(1):5199. doi: 10.1038/s41598-021-84548-4. Sci Rep. 2021. PMID: 33664344 Free PMC article.
-
Essential role of class II phosphatidylinositol-3-kinase-C2α in sphingosine 1-phosphate receptor-1-mediated signaling and migration in endothelial cells.J Biol Chem. 2013 Jan 25;288(4):2325-39. doi: 10.1074/jbc.M112.409656. Epub 2012 Nov 28. J Biol Chem. 2013. PMID: 23192342 Free PMC article.
-
TGFβ receptor endocytosis and Smad signaling require synaptojanin1, PI3K-C2α-, and INPP4B-mediated phosphoinositide conversions.Mol Biol Cell. 2020 Mar 1;31(5):360-372. doi: 10.1091/mbc.E19-11-0662. Epub 2020 Jan 8. Mol Biol Cell. 2020. PMID: 31913757 Free PMC article.
-
Smad anchor for receptor activation (SARA) in TGF-beta signaling.Front Biosci (Elite Ed). 2010 Jun 1;2(3):857-60. doi: 10.2741/e147. Front Biosci (Elite Ed). 2010. PMID: 20515759 Review.
-
PI3K-C2α: One enzyme for two products coupling vesicle trafficking and signal transduction.FEBS Lett. 2015 Jun 22;589(14):1552-8. doi: 10.1016/j.febslet.2015.05.001. Epub 2015 May 12. FEBS Lett. 2015. PMID: 25979177 Review.
Cited by
-
Lymphocytic, cytokine and transcriptomic profiles in peripheral blood of dogs with atopic dermatitis.BMC Vet Res. 2016 Aug 23;12(1):174. doi: 10.1186/s12917-016-0805-6. BMC Vet Res. 2016. PMID: 27553600 Free PMC article.
-
Kaposi's Sarcoma-Associated Herpesvirus Nonstructural Membrane Protein pK15 Recruits the Class II Phosphatidylinositol 3-Kinase PI3K-C2α To Activate Productive Viral Replication.J Virol. 2018 Aug 16;92(17):e00544-18. doi: 10.1128/JVI.00544-18. Print 2018 Sep 1. J Virol. 2018. PMID: 29950425 Free PMC article.
-
PI3K Isoforms in Vascular Biology, A Focus on the Vascular System-Immune Response Connection.Curr Top Microbiol Immunol. 2022;436:289-309. doi: 10.1007/978-3-031-06566-8_12. Curr Top Microbiol Immunol. 2022. PMID: 36243849
-
PI3K-C2α knockdown decreases autophagy and maturation of endocytic vesicles.PLoS One. 2017 Sep 14;12(9):e0184909. doi: 10.1371/journal.pone.0184909. eCollection 2017. PLoS One. 2017. PMID: 28910396 Free PMC article.
-
MicroRNA-744 Inhibits Proliferation of Bronchial Epithelial Cells by Regulating Smad3 Pathway via Targeting Transforming Growth Factor-β1 (TGF-β1) in Severe Asthma.Med Sci Monit. 2019 Mar 23;25:2159-2168. doi: 10.12659/MSM.912412. Med Sci Monit. 2019. PMID: 30903795 Free PMC article.
References
-
- Engelman J. A., Luo J., Cantley L. C. (2006) The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 7, 606–619 - PubMed
-
- Vanhaesebroeck B., Guillermet-Guibert J., Graupera M., Bilanges B. (2010) The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 11, 329–341 - PubMed
-
- Virbasius J. V., Guilherme A., Czech M. P. (1996) Mouse p170 is a novel phosphatidylinositol 3-kinase containing a C2 domain. J. Biol. Chem. 271, 13304–13307 - PubMed
-
- Molz L., Chen Y. W., Hirano M., Williams L. T. (1996) Cpk is a novel class of Drosophila PtdIns 3-kinase containing a C2 domain. J. Biol. Chem. 271, 13892–13899 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

