The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease
- PMID: 25611507
- PMCID: PMC4764997
- DOI: 10.1016/j.neuron.2014.12.007
The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease
Abstract
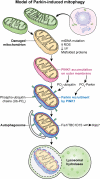
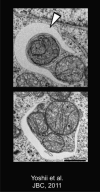
Understanding the function of genes mutated in hereditary forms of Parkinson's disease yields insight into disease etiology and reveals new pathways in cell biology. Although mutations or variants in many genes increase the susceptibility to Parkinson's disease, only a handful of monogenic causes of parkinsonism have been identified. Biochemical and genetic studies reveal that the products of two genes that are mutated in autosomal recessive parkinsonism, PINK1 and Parkin, normally work together in the same pathway to govern mitochondrial quality control, bolstering previous evidence that mitochondrial damage is involved in Parkinson's disease. PINK1 accumulates on the outer membrane of damaged mitochondria, activates Parkin's E3 ubiquitin ligase activity, and recruits Parkin to the dysfunctional mitochondrion. Then, Parkin ubiquitinates outer mitochondrial membrane proteins to trigger selective autophagy. This review covers the normal functions that PINK1 and Parkin play within cells, their molecular mechanisms of action, and the pathophysiological consequences of their loss.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
N-degron-mediated degradation and regulation of mitochondrial PINK1 kinase.Curr Genet. 2020 Aug;66(4):693-701. doi: 10.1007/s00294-020-01062-2. Epub 2020 Mar 10. Curr Genet. 2020. PMID: 32157382 Review.
-
Deciphering the Molecular Signals of PINK1/Parkin Mitophagy.Trends Cell Biol. 2016 Oct;26(10):733-744. doi: 10.1016/j.tcb.2016.05.008. Epub 2016 Jun 10. Trends Cell Biol. 2016. PMID: 27291334 Review.
-
Parkin and PINK1 functions in oxidative stress and neurodegeneration.Brain Res Bull. 2017 Jul;133:51-59. doi: 10.1016/j.brainresbull.2016.12.004. Epub 2016 Dec 23. Brain Res Bull. 2017. PMID: 28017782 Free PMC article. Review.
-
The mitochondrial kinase PINK1: functions beyond mitophagy.J Neurochem. 2016 Oct;139 Suppl 1:232-239. doi: 10.1111/jnc.13655. Epub 2016 Jun 2. J Neurochem. 2016. PMID: 27251035 Review.
-
Mitochondrial quality control mediated by PINK1 and Parkin: links to parkinsonism.Cold Spring Harb Perspect Biol. 2012 Nov 1;4(11):a011338. doi: 10.1101/cshperspect.a011338. Cold Spring Harb Perspect Biol. 2012. PMID: 23125018 Free PMC article.
Cited by
-
COVID-19 and neurodegeneration: The mitochondrial connection.Aging Cell. 2022 Nov;21(11):e13727. doi: 10.1111/acel.13727. Epub 2022 Oct 11. Aging Cell. 2022. PMID: 36219531 Free PMC article.
-
Absence of the Autophagy Adaptor SQSTM1/p62 Causes Childhood-Onset Neurodegeneration with Ataxia, Dystonia, and Gaze Palsy.Am J Hum Genet. 2016 Sep 1;99(3):735-743. doi: 10.1016/j.ajhg.2016.06.026. Epub 2016 Aug 18. Am J Hum Genet. 2016. PMID: 27545679 Free PMC article.
-
Mitochondrial dysfunction in Parkinson's disease - a key disease hallmark with therapeutic potential.Mol Neurodegener. 2023 Nov 11;18(1):83. doi: 10.1186/s13024-023-00676-7. Mol Neurodegener. 2023. PMID: 37951933 Free PMC article. Review.
-
Gut microbiome and Parkinson's disease: Perspective on pathogenesis and treatment.J Adv Res. 2023 Aug;50:83-105. doi: 10.1016/j.jare.2022.10.013. Epub 2022 Nov 1. J Adv Res. 2023. PMID: 36332796 Free PMC article. Review.
-
Basal Gp78-dependent mitophagy promotes mitochondrial health and limits mitochondrial ROS.Cell Mol Life Sci. 2022 Oct 25;79(11):565. doi: 10.1007/s00018-022-04585-8. Cell Mol Life Sci. 2022. PMID: 36284011 Free PMC article.
References
-
- Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. - PubMed
-
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

