Rare variants in RTEL1 are associated with familial interstitial pneumonia
- PMID: 25607374
- PMCID: PMC4384777
- DOI: 10.1164/rccm.201408-1510OC
Rare variants in RTEL1 are associated with familial interstitial pneumonia
Abstract
Rationale: Up to 20% of cases of idiopathic interstitial pneumonia cluster in families, comprising the syndrome of familial interstitial pneumonia (FIP); however, the genetic basis of FIP remains uncertain in most families.
Objectives: To determine if new disease-causing rare genetic variants could be identified using whole-exome sequencing of affected members from FIP families, providing additional insights into disease pathogenesis.
Methods: Affected subjects from 25 kindreds were selected from an ongoing FIP registry for whole-exome sequencing from genomic DNA. Candidate rare variants were confirmed by Sanger sequencing, and cosegregation analysis was performed in families, followed by additional sequencing of affected individuals from another 163 kindreds.
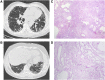
Measurements and main results: We identified a potentially damaging rare variant in the gene encoding for regulator of telomere elongation helicase 1 (RTEL1) that segregated with disease and was associated with very short telomeres in peripheral blood mononuclear cells in 1 of 25 families in our original whole-exome sequencing cohort. Evaluation of affected individuals in 163 additional kindreds revealed another eight families (4.7%) with heterozygous rare variants in RTEL1 that segregated with clinical FIP. Probands and unaffected carriers of these rare variants had short telomeres (<10% for age) in peripheral blood mononuclear cells and increased T-circle formation, suggesting impaired RTEL1 function.
Conclusions: Rare loss-of-function variants in RTEL1 represent a newly defined genetic predisposition for FIP, supporting the importance of telomere-related pathways in pulmonary fibrosis.
Keywords: genetics; idiopathic pulmonary fibrosis; telomere.
Figures

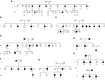


Comment in
-
What the genetics "RTEL"ing us about telomeres and pulmonary fibrosis.Am J Respir Crit Care Med. 2015 Mar 15;191(6):608-10. doi: 10.1164/rccm.201501-0119ED. Am J Respir Crit Care Med. 2015. PMID: 25767920 Free PMC article. No abstract available.
Similar articles
-
Regulator of telomere length 1 (RTEL1) mutations are associated with heterogeneous pulmonary and extra-pulmonary phenotypes.Eur Respir J. 2019 Feb 7;53(2):1800508. doi: 10.1183/13993003.00508-2018. Print 2019 Feb. Eur Respir J. 2019. PMID: 30523160
-
Three-dimensional spatial analysis of missense variants in RTEL1 identifies pathogenic variants in patients with Familial Interstitial Pneumonia.BMC Bioinformatics. 2018 Jan 23;19(1):18. doi: 10.1186/s12859-018-2010-z. BMC Bioinformatics. 2018. PMID: 29361909 Free PMC article.
-
Heterozygous RTEL1 mutations are associated with familial pulmonary fibrosis.Eur Respir J. 2015 Aug;46(2):474-85. doi: 10.1183/09031936.00040115. Epub 2015 May 28. Eur Respir J. 2015. PMID: 26022962
-
Pulmonary fibrosis in the era of stratified medicine.Thorax. 2016 Dec;71(12):1154-1160. doi: 10.1136/thoraxjnl-2016-209172. Epub 2016 Oct 31. Thorax. 2016. PMID: 27799632 Free PMC article. Review.
-
The many faces of the helicase RTEL1 at telomeres and beyond.Trends Cell Biol. 2024 Feb;34(2):109-121. doi: 10.1016/j.tcb.2023.07.002. Epub 2023 Jul 31. Trends Cell Biol. 2024. PMID: 37532653 Review.
Cited by
-
Synonymous Mutation in DKC1 Causes Telomerase RNA Insufficiency Manifesting as Familial Pulmonary Fibrosis.Chest. 2020 Dec;158(6):2449-2457. doi: 10.1016/j.chest.2020.07.025. Epub 2020 Jul 22. Chest. 2020. PMID: 32710892 Free PMC article.
-
Genome-wide imputation study identifies novel HLA locus for pulmonary fibrosis and potential role for auto-immunity in fibrotic idiopathic interstitial pneumonia.BMC Genet. 2016 Jun 7;17(1):74. doi: 10.1186/s12863-016-0377-2. BMC Genet. 2016. PMID: 27266705 Free PMC article.
-
Pilot experience of multidisciplinary team discussion dedicated to inherited pulmonary fibrosis.Orphanet J Rare Dis. 2019 Dec 3;14(1):280. doi: 10.1186/s13023-019-1256-5. Orphanet J Rare Dis. 2019. PMID: 31796085 Free PMC article.
-
Idiopathic pulmonary fibrosis: Epithelial-mesenchymal interactions and emerging therapeutic targets.Matrix Biol. 2018 Oct;71-72:112-127. doi: 10.1016/j.matbio.2018.03.021. Epub 2018 Apr 3. Matrix Biol. 2018. PMID: 29625182 Free PMC article. Review.
-
Aging and Lung Disease. Clinical Impact and Cellular and Molecular Pathways.Ann Am Thorac Soc. 2015 Dec;12(12):S222-7. doi: 10.1513/AnnalsATS.201508-484PL. Ann Am Thorac Soc. 2015. PMID: 26653202 Free PMC article. Review.
References
-
- King TE, Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–1961. - PubMed
-
- Garcia-Sancho C, Buendia-Roldan I, Fernandez-Plata MR, Navarro C, Perez-Padilla R, Vargas MH, Loyd JE, Selman M. Familial pulmonary fibrosis is the strongest risk factor for idiopathic pulmonary fibrosis. Respir Med. 2011;105:1902–1907. - PubMed
-
- Nogee LM, Dunbar AE, III, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344:573–579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL085317/HL/NHLBI NIH HHS/United States
- HL92870/HL/NHLBI NIH HHS/United States
- U01 HG007674/HG/NHGRI NIH HHS/United States
- R01 HL105479/HL/NHLBI NIH HHS/United States
- T32 HL094296/HL/NHLBI NIH HHS/United States
- R01 HL085317/HL/NHLBI NIH HHS/United States
- I01 BX001988/BX/BLRD VA/United States
- HL094296/HL/NHLBI NIH HHS/United States
- UM1 HG006493/HG/NHGRI NIH HHS/United States
- R01 HL097163/HL/NHLBI NIH HHS/United States
- HL0097163/HL/NHLBI NIH HHS/United States
- R01 HL119503/HL/NHLBI NIH HHS/United States
- P01 HL092870/HL/NHLBI NIH HHS/United States
- U54 HG006493/HG/NHGRI NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
- 1U54HG006493/HG/NHGRI NIH HHS/United States
- R33 HL120770/HL/NHLBI NIH HHS/United States
- HL105479/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

