Vaccinia virus protein A49 is an unexpected member of the B-cell Lymphoma (Bcl)-2 protein family
- PMID: 25605733
- PMCID: PMC4358236
- DOI: 10.1074/jbc.M114.624650
Vaccinia virus protein A49 is an unexpected member of the B-cell Lymphoma (Bcl)-2 protein family
Abstract
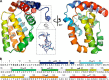
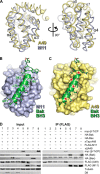
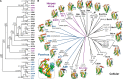
Vaccinia virus (VACV) encodes several proteins that inhibit activation of the proinflammatory transcription factor nuclear factor κB (NF-κB). VACV protein A49 prevents translocation of NF-κB to the nucleus by sequestering cellular β-TrCP, a protein required for the degradation of the inhibitor of κB. A49 does not share overall sequence similarity with any protein of known structure or function. We solved the crystal structure of A49 from VACV Western Reserve to 1.8 Å resolution and showed, surprisingly, that A49 has the same three-dimensional fold as Bcl-2 family proteins despite lacking identifiable sequence similarity. Whereas Bcl-2 family members characteristically modulate cellular apoptosis, A49 lacks a surface groove suitable for binding BH3 peptides and does not bind proapoptotic Bcl-2 family proteins Bax or Bak. The N-terminal 17 residues of A49 do not adopt a single well ordered conformation, consistent with their proposed role in binding β-TrCP. Whereas pairs of A49 molecules interact symmetrically via a large hydrophobic surface in crystallo, A49 does not dimerize in solution or in cells, and we propose that this hydrophobic interaction surface may mediate binding to a yet undefined cellular partner. A49 represents the eleventh VACV Bcl-2 family protein and, despite these proteins sharing very low sequence identity, structure-based phylogenetic analysis shows that all poxvirus Bcl-2 proteins are structurally more similar to each other than they are to any cellular or herpesvirus Bcl-2 proteins. This is consistent with duplication and diversification of a single BCL2 family gene acquired by an ancestral poxvirus.
Keywords: B-cell Lymphoma 2 (Bcl-2) Family; Innate Immunity; NF-κB (NF-KB); Poxvirus; Protein Structure; Structure-based Phylogenetics; Vaccinia Virus; Viral Protein.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
Leaky scanning translation generates a second A49 protein that contributes to vaccinia virus virulence.J Gen Virol. 2020 May;101(5):533-541. doi: 10.1099/jgv.0.001386. Epub 2020 Feb 24. J Gen Virol. 2020. PMID: 32100702 Free PMC article.
-
Vaccinia virus proteins A52 and B14 Share a Bcl-2-like fold but have evolved to inhibit NF-kappaB rather than apoptosis.PLoS Pathog. 2008 Aug 15;4(8):e1000128. doi: 10.1371/journal.ppat.1000128. PLoS Pathog. 2008. PMID: 18704168 Free PMC article.
-
NF-κB activation is a turn on for vaccinia virus phosphoprotein A49 to turn off NF-κB activation.Proc Natl Acad Sci U S A. 2019 Mar 19;116(12):5699-5704. doi: 10.1073/pnas.1813504116. Epub 2019 Feb 28. Proc Natl Acad Sci U S A. 2019. PMID: 30819886 Free PMC article.
-
Structural biology of the Bcl-2 family of proteins.Biochim Biophys Acta. 2004 Mar 1;1644(2-3):83-94. doi: 10.1016/j.bbamcr.2003.08.012. Biochim Biophys Acta. 2004. PMID: 14996493 Review.
-
Structural biology of the Bcl-2 family and its mimicry by viral proteins.Cell Death Dis. 2013 Nov 7;4(11):e909. doi: 10.1038/cddis.2013.436. Cell Death Dis. 2013. PMID: 24201808 Free PMC article. Review.
Cited by
-
Cullin-5 Adaptor SPSB1 Controls NF-κB Activation Downstream of Multiple Signaling Pathways.Front Immunol. 2020 Jan 21;10:3121. doi: 10.3389/fimmu.2019.03121. eCollection 2019. Front Immunol. 2020. PMID: 32038638 Free PMC article.
-
VPS18 recruits VPS41 to the human HOPS complex via a RING-RING interaction.Biochem J. 2017 Oct 23;474(21):3615-3626. doi: 10.1042/BCJ20170588. Biochem J. 2017. PMID: 28931724 Free PMC article.
-
Leaky scanning translation generates a second A49 protein that contributes to vaccinia virus virulence.J Gen Virol. 2020 May;101(5):533-541. doi: 10.1099/jgv.0.001386. Epub 2020 Feb 24. J Gen Virol. 2020. PMID: 32100702 Free PMC article.
-
The Bcl-2 Family in Host-Virus Interactions.Viruses. 2017 Oct 6;9(10):290. doi: 10.3390/v9100290. Viruses. 2017. PMID: 28984827 Free PMC article. Review.
-
pUL21 is a viral phosphatase adaptor that promotes herpes simplex virus replication and spread.PLoS Pathog. 2021 Aug 16;17(8):e1009824. doi: 10.1371/journal.ppat.1009824. eCollection 2021 Aug. PLoS Pathog. 2021. PMID: 34398933 Free PMC article.
References
-
- Moss B. (2007) in Fields' Virology, 5th Ed (Fields B. N., Knipe D. M., Howley P. M., eds) pp. 2905–2946, Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia
-
- Gubser C., Hué S., Kellam P., Smith G. L. (2004) Poxvirus genomes: a phylogenetic analysis. J. Gen. Virol. 85, 105–117 - PubMed
-
- Mansur D. S., Maluquer de Motes C., Unterholzner L., Sumner R. P., Ferguson B. J., Ren H., Strnadova P., Bowie A. G., Smith G. L. (2013) Poxvirus targeting of E3 ligase β-TrCP by molecular mimicry: a mechanism to inhibit NF-κB activation and promote immune evasion and virulence. PLoS Pathog. 9, e1003183. - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

