Chromatinization of the KSHV Genome During the KSHV Life Cycle
- PMID: 25594667
- PMCID: PMC4381254
- DOI: 10.3390/cancers7010112
Chromatinization of the KSHV Genome During the KSHV Life Cycle
Abstract
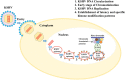
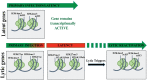
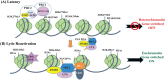
Kaposi's sarcoma-associated herpesvirus (KSHV) belongs to the gamma herpesvirus family and is the causative agent of various lymphoproliferative diseases in humans. KSHV, like other herpesviruses, establishes life-long latent infection with the expression of a limited number of viral genes. Expression of these genes is tightly regulated by both the viral and cellular factors. Recent advancements in identifying the expression profiles of viral transcripts, using tilling arrays and next generation sequencing have identified additional coding and non-coding transcripts in the KSHV genome. Determining the functions of these transcripts will provide a better understanding of the mechanisms utilized by KSHV in altering cellular pathways involved in promoting cell growth and tumorigenesis. Replication of the viral genome is critical in maintaining the existing copies of the viral episomes during both latent and lytic phases of the viral life cycle. The replication of the viral episome is facilitated by viral components responsible for recruiting chromatin modifying enzymes and replication factors for altering the chromatin complexity and replication initiation functions, respectively. Importantly, chromatin modification of the viral genome plays a crucial role in determining whether the viral genome will persist as latent episome or undergo lytic reactivation. Additionally, chromatinization of the incoming virion DNA, which lacks chromatin structure, in the target cells during primary infection, helps in establishing latent infection. Here, we discuss the recent advancements on our understating of KSHV genome chromatinization and the consequences of chromatin modifications on viral life cycle.
Figures
Similar articles
-
Transcriptome analysis of Kaposi's sarcoma-associated herpesvirus during de novo primary infection of human B and endothelial cells.J Virol. 2015 Mar;89(6):3093-111. doi: 10.1128/JVI.02507-14. Epub 2014 Dec 31. J Virol. 2015. PMID: 25552714 Free PMC article.
-
ARID3B: a Novel Regulator of the Kaposi's Sarcoma-Associated Herpesvirus Lytic Cycle.J Virol. 2016 Sep 29;90(20):9543-55. doi: 10.1128/JVI.03262-15. Print 2016 Oct 15. J Virol. 2016. PMID: 27512077 Free PMC article.
-
Complex Interactions between Cohesin and CTCF in Regulation of Kaposi's Sarcoma-Associated Herpesvirus Lytic Transcription.J Virol. 2020 Jan 6;94(2):e01279-19. doi: 10.1128/JVI.01279-19. Print 2020 Jan 6. J Virol. 2020. PMID: 31666380 Free PMC article.
-
Reactivation and Lytic Replication of Kaposi's Sarcoma-Associated Herpesvirus: An Update.Front Microbiol. 2017 Apr 20;8:613. doi: 10.3389/fmicb.2017.00613. eCollection 2017. Front Microbiol. 2017. PMID: 28473805 Free PMC article. Review.
-
KSHV Genome Replication and Maintenance.Front Microbiol. 2016 Feb 1;7:54. doi: 10.3389/fmicb.2016.00054. eCollection 2016. Front Microbiol. 2016. PMID: 26870016 Free PMC article. Review.
Cited by
-
PRC1-independent binding and activity of RYBP on the KSHV genome during de novo infection.PLoS Pathog. 2022 Aug 26;18(8):e1010801. doi: 10.1371/journal.ppat.1010801. eCollection 2022 Aug. PLoS Pathog. 2022. PMID: 36026503 Free PMC article.
-
Kaposi's Sarcoma-Associated Herpesvirus Processivity Factor, ORF59, Binds to Canonical and Linker Histones, and Its Carboxy Terminus Is Dispensable for Viral DNA Synthesis.J Virol. 2021 Feb 24;95(6):e02169-20. doi: 10.1128/JVI.02169-20. Print 2021 Feb 24. J Virol. 2021. PMID: 33361421 Free PMC article.
-
KSHV-Mediated Regulation of Par3 and SNAIL Contributes to B-Cell Proliferation.PLoS Pathog. 2016 Jul 27;12(7):e1005801. doi: 10.1371/journal.ppat.1005801. eCollection 2016 Jul. PLoS Pathog. 2016. PMID: 27463802 Free PMC article.
-
Kaposi's Sarcoma-Associated Herpesvirus ORF66 Is Essential for Late Gene Expression and Virus Production via Interaction with ORF34.J Virol. 2020 Jan 6;94(2):e01300-19. doi: 10.1128/JVI.01300-19. Print 2020 Jan 6. J Virol. 2020. PMID: 31694948 Free PMC article.
-
Insight into the Epigenetics of Kaposi's Sarcoma-Associated Herpesvirus.Int J Mol Sci. 2023 Oct 6;24(19):14955. doi: 10.3390/ijms241914955. Int J Mol Sci. 2023. PMID: 37834404 Free PMC article. Review.
References
-
- Soulier J., Grollet L., Oksenhendler E., Cacoub P., Cazals-Hatem D., Babinet P., dʼAgay M.F., Clauvel J.P., Raphael M., Degos L., et al. Kaposiʼs sarcoma-associated herpesvirus-like DNA sequences in multicentric castlemanʼs disease. Blood. 1995;86:1276–1280. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

