Mesenchymal stem cells detect and defend against gammaherpesvirus infection via the cGAS-STING pathway
- PMID: 25592282
- PMCID: PMC4296288
- DOI: 10.1038/srep07820
Mesenchymal stem cells detect and defend against gammaherpesvirus infection via the cGAS-STING pathway
Abstract
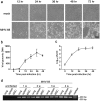
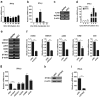
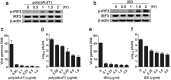
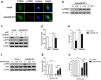
Mesenchymal stem cells (MSCs) are widely used in clinical settings to treat tissue injuries and autoimmune disorders due to their multipotentiality and immunomodulation. Long-term observations reveal several complications after MSCs infusion, especially herpesviral infection. However, the mechanism of host defense against herpesviruses in MSCs remains largely unknown. Here we showed that murine gammaherpesvirus-68 (MHV-68), which is genetically and biologically related to human gammaherpesviruses, efficiently infected MSCs both in vitro and in vivo. Cytosolic DNA sensor cyclic GMP-AMP synthase (cGAS) was identified as the sensor of MHV-68 in MSCs for the first time. Moreover, the cytosolic DNA sensing pathway mediated a potent anti-herpesviral effect through the adaptor STING and downstream kinase TBK1. Furthermore, blockade of IFN signaling suggested that cytosolic DNA sensing triggered both IFN-dependent and -independent anti-herpesviral responses. Our findings demonstrate that cGAS-STING mediates innate immunity to gammaherpesvirus infection in MSCs, which may provide a clue to develop therapeutic strategy.
Figures
Similar articles
-
Modulation of the cGAS-STING DNA sensing pathway by gammaherpesviruses.Proc Natl Acad Sci U S A. 2015 Aug 4;112(31):E4306-15. doi: 10.1073/pnas.1503831112. Epub 2015 Jul 21. Proc Natl Acad Sci U S A. 2015. PMID: 26199418 Free PMC article.
-
African Swine Fever Virus Armenia/07 Virulent Strain Controls Interferon Beta Production through the cGAS-STING Pathway.J Virol. 2019 May 29;93(12):e02298-18. doi: 10.1128/JVI.02298-18. Print 2019 Jun 15. J Virol. 2019. PMID: 30918080 Free PMC article.
-
Herpes Simplex Virus 1 Abrogates the cGAS/STING-Mediated Cytosolic DNA-Sensing Pathway via Its Virion Host Shutoff Protein, UL41.J Virol. 2017 Feb 28;91(6):e02414-16. doi: 10.1128/JVI.02414-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28077645 Free PMC article.
-
Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing.Nat Immunol. 2016 Sep 20;17(10):1142-9. doi: 10.1038/ni.3558. Nat Immunol. 2016. PMID: 27648547 Review.
-
Crosstalk between cGAS-STING signaling and cell death.Cell Death Differ. 2020 Nov;27(11):2989-3003. doi: 10.1038/s41418-020-00624-8. Epub 2020 Sep 18. Cell Death Differ. 2020. PMID: 32948836 Free PMC article. Review.
Cited by
-
Long-term safety of umbilical cord mesenchymal stem cells transplantation for systemic lupus erythematosus: a 6-year follow-up study.Clin Exp Med. 2017 Aug;17(3):333-340. doi: 10.1007/s10238-016-0427-0. Epub 2016 Jun 7. Clin Exp Med. 2017. PMID: 27270729
-
Clinical efficacy and mechanism of mesenchymal stromal cells in treatment of COVID-19.Stem Cell Res Ther. 2022 Feb 7;13(1):61. doi: 10.1186/s13287-022-02743-0. Stem Cell Res Ther. 2022. PMID: 35130977 Free PMC article. Review.
-
Bone marrow mesenchymal stem cell aggregate: an optimal cell therapy for full-layer cutaneous wound vascularization and regeneration.Sci Rep. 2015 Nov 23;5:17036. doi: 10.1038/srep17036. Sci Rep. 2015. PMID: 26594024 Free PMC article.
-
Glucocorticoids Suppress Antimicrobial Autophagy and Nitric Oxide Production and Facilitate Mycobacterial Survival in Macrophages.Sci Rep. 2017 Apr 20;7(1):982. doi: 10.1038/s41598-017-01174-9. Sci Rep. 2017. PMID: 28428627 Free PMC article.
-
Two to Tango: Dialog between Immunity and Stem Cells in Health and Disease.Cell. 2018 Nov 1;175(4):908-920. doi: 10.1016/j.cell.2018.08.071. Cell. 2018. PMID: 30388451 Free PMC article. Review.
References
-
- Uccelli A., Moretta L. & Pistoia V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 8, 726–736 (2008). - PubMed
-
- Chou S. H. et al. Mesenchymal stem cell insights: prospects in hematological transplantation. Cell Transplant. 22, 711–721 (2013). - PubMed
-
- Le Blanc K. et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363, 1439–1441 (2004). - PubMed
-
- Bernardo M. E. & Fibbe W. E. Safety and efficacy of mesenchymal stromal cell therapy in autoimmune disorders. Ann. N. Y. Acad. Sci. 1266, 107–117 (2012). - PubMed
-
- von Bahr L. et al. Long-term complications, immunologic effects, and role of passage for outcome in mesenchymal stromal cell therapy. Biol. Blood Marrow Transplant. 18, 557–564 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

