LAMC2 enhances the metastatic potential of lung adenocarcinoma
- PMID: 25591736
- PMCID: PMC4495359
- DOI: 10.1038/cdd.2014.228
LAMC2 enhances the metastatic potential of lung adenocarcinoma
Abstract
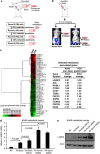
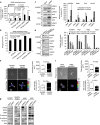
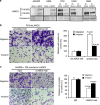
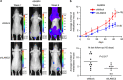
Lung cancer is the number one cancer killer, and metastasis is the main cause of high mortality in lung cancer patients. However, mechanisms underlying the development of lung cancer metastasis remain unknown. Using genome-wide transcriptional analysis in an experimental metastasis model, we identified laminin γ2 (LAMC2), an epithelial basement membrane protein, to be significantly upregulated in lung adenocarcinoma metastatic cells. Elevated LAMC2 increased traction force, migration, and invasion of lung adenocarcinoma cells accompanied by the induction of epithelial-mesenchymal transition (EMT). LAMC2 knockdown decreased traction force, migration, and invasion accompanied by EMT reduction in vitro, and attenuated metastasis in mice. LAMC2 promoted migration and invasion via EMT that was integrin β1- and ZEB1-dependent. High LAMC2 was significantly correlated with the mesenchymal marker vimentin expression in lung adenocarcinomas, and with higher risk of recurrence or death in patients with lung adenocarcinoma. We suggest that LAMC2 promotes metastasis in lung adenocarcinoma via EMT and may be a potential therapeutic target.
Figures


Similar articles
-
37-kDa laminin receptor precursor promotes lung adenocarcinoma cell invasion and metastasis by epithelial-to-mesenchymal transition.Cancer Gene Ther. 2014 Apr;21(4):150-7. doi: 10.1038/cgt.2014.10. Epub 2014 Apr 11. Cancer Gene Ther. 2014. PMID: 24722356
-
Extracellular matrix marker LAMC2 targets ZEB1 to promote TNBC malignancy via up-regulating CD44/STAT3 signaling pathway.Mol Med. 2024 May 17;30(1):61. doi: 10.1186/s10020-024-00827-6. Mol Med. 2024. PMID: 38760717 Free PMC article.
-
Overexpression of laminin-5 gamma-2 promotes tumorigenesis of pancreatic ductal adenocarcinoma through EGFR/ERK1/2/AKT/mTOR cascade.Cell Mol Life Sci. 2022 Jun 14;79(7):362. doi: 10.1007/s00018-022-04392-1. Cell Mol Life Sci. 2022. PMID: 35699794 Free PMC article.
-
Geraniin inhibits TGF-β1-induced epithelial-mesenchymal transition and suppresses A549 lung cancer migration, invasion and anoikis resistance.Bioorg Med Chem Lett. 2015 Sep 1;25(17):3529-34. doi: 10.1016/j.bmcl.2015.06.093. Epub 2015 Jul 4. Bioorg Med Chem Lett. 2015. PMID: 26169124
-
Involvement of LKB1 in epithelial-mesenchymal transition (EMT) of human lung cancer cells.Lung Cancer. 2010 Nov;70(2):136-45. doi: 10.1016/j.lungcan.2010.02.004. Epub 2010 Mar 5. Lung Cancer. 2010. PMID: 20207041
Cited by
-
Epithelial-mesenchymal plasticity (EMP) in wound healing: Exploring EMT mechanisms, regulatory network, and therapeutic opportunities.Heliyon. 2024 Jul 8;10(14):e34269. doi: 10.1016/j.heliyon.2024.e34269. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39108889 Free PMC article. Review.
-
A genetic cell context-dependent role for ZEB1 in lung cancer.Nat Commun. 2016 Jul 26;7:12231. doi: 10.1038/ncomms12231. Nat Commun. 2016. PMID: 27456471 Free PMC article.
-
Highly sensitive detection of invasive lung cancer cells by novel antibody against amino-terminal domain of laminin γ2 chain.Cancer Sci. 2016 Dec;107(12):1909-1918. doi: 10.1111/cas.13089. Epub 2016 Dec 12. Cancer Sci. 2016. PMID: 27685891 Free PMC article.
-
Serum LAMC2 enhances the prognostic value of a multi-parametric panel in non-small cell lung cancer.Br J Cancer. 2015 Jul 28;113(3):484-91. doi: 10.1038/bjc.2015.171. Epub 2015 Jul 16. Br J Cancer. 2015. PMID: 26180921 Free PMC article.
-
Broad and thematic remodeling of the surfaceome and glycoproteome on isogenic cells transformed with driving proliferative oncogenes.Proc Natl Acad Sci U S A. 2020 Apr 7;117(14):7764-7775. doi: 10.1073/pnas.1917947117. Epub 2020 Mar 23. Proc Natl Acad Sci U S A. 2020. PMID: 32205440 Free PMC article.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. Cancer J Clin. 2013;63:11–30. - PubMed
-
- Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn. 2000;218:213–234. - PubMed
-
- Moriya Y, Niki T, Yamada T, Matsuno Y, Kondo H, Hirohashi S. Increased expression of laminin-5 and its prognostic significance in lung adenocarcinomas of small size. An immunohistochemical analysis of 102 cases. Cancer. 2001;91:1129–1141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

