Neuronal cell cycle: the neuron itself and its circumstances
- PMID: 25590687
- PMCID: PMC4418291
- DOI: 10.1080/15384101.2015.1004937
Neuronal cell cycle: the neuron itself and its circumstances
Abstract
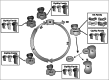
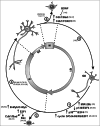
Neurons are usually regarded as postmitotic cells that undergo apoptosis in response to cell cycle reactivation. Nevertheless, recent evidence indicates the existence of a defined developmental program that induces DNA replication in specific populations of neurons, which remain in a tetraploid state for the rest of their adult life. Similarly, de novo neuronal tetraploidization has also been described in the adult brain as an early hallmark of neurodegeneration. The aim of this review is to integrate these recent developments in the context of cell cycle regulation and apoptotic cell death in neurons. We conclude that a variety of mechanisms exists in neuronal cells for G1/S and G2/M checkpoint regulation. These mechanisms, which are connected with the apoptotic machinery, can be modulated by environmental signals and the neuronal phenotype itself, thus resulting in a variety of outcomes ranging from cell death at the G1/S checkpoint to full proliferation of differentiated neurons.
Keywords: AD, Alzheimer disease; BDNF, brain-derived neurotrophic factor; BrdU, 5-bromo-2′-deoxyuridine; CKI, Cdk-inhibitor; CNS, central nervous system; Cdk, cyclin-dependent kinase; Cip/Kip, cyclin inhibitor protein/kinase inhibitor protein; G0, quiescent state; G1, growth phase 1; G2, growth phase 2; Ink, inhibitor of kinase; Mcm2, minichromosome maintenance 2; PCNA, proliferating cell nuclear antigen; PD, Parkinson disease; RGCs, retinal ganglion cells; Rb, Retinoblastoma; S-phase; S-phase, synthesis phase.; apoptosis; cell cycle re-entry; mitosis; neuron; p38MAPK, p38 mitogen-activated protein kinase; p75NTR, neurotrophin receptor p75; tetraploid.
Figures
Similar articles
-
A novel hypothesis for Alzheimer disease based on neuronal tetraploidy induced by p75 (NTR).Cell Cycle. 2010 May 15;9(10):1934-41. doi: 10.4161/cc.9.10.11582. Epub 2010 May 15. Cell Cycle. 2010. PMID: 20436277 Review.
-
Neuronal apoptosis at the G1/S cell cycle checkpoint.Cell Tissue Res. 2001 Aug;305(2):217-28. doi: 10.1007/s004410100396. Cell Tissue Res. 2001. PMID: 11545259 Review.
-
Coordination of proliferation and neuronal differentiation by the retinoblastoma protein family.Dev Growth Differ. 2014 Jun;56(5):324-34. doi: 10.1111/dgd.12127. Epub 2014 Apr 3. Dev Growth Differ. 2014. PMID: 24697649 Review.
-
Id1 and Sonic Hedgehog Mediate Cell Cycle Reentry and Apoptosis Induced by Amyloid Beta-Peptide in Post-mitotic Cortical Neurons.Mol Neurobiol. 2019 Jan;56(1):465-489. doi: 10.1007/s12035-018-1098-5. Epub 2018 May 2. Mol Neurobiol. 2019. PMID: 29721855
-
Hypoxia-ischemia induces DNA synthesis without cell proliferation in dying neurons in adult rodent brain.J Neurosci. 2004 Nov 24;24(47):10763-72. doi: 10.1523/JNEUROSCI.3883-04.2004. J Neurosci. 2004. PMID: 15564594 Free PMC article.
Cited by
-
Cell Cycle Deficits in Neurodegenerative Disorders: Uncovering Molecular Mechanisms to Drive Innovative Therapeutic Development.Aging Dis. 2020 Jul 23;11(4):946-966. doi: 10.14336/AD.2019.0923. eCollection 2020 Jul. Aging Dis. 2020. PMID: 32765956 Free PMC article. Review.
-
E2F4DN Transgenic Mice: A Tool for the Evaluation of E2F4 as a Therapeutic Target in Neuropathology and Brain Aging.Int J Mol Sci. 2022 Oct 11;23(20):12093. doi: 10.3390/ijms232012093. Int J Mol Sci. 2022. PMID: 36292945 Free PMC article. Review.
-
Genetically Encoded and Modular SubCellular Organelle Probes (GEM-SCOPe) reveal lysosomal and mitochondrial dysfunction driven by PRKN knockout.bioRxiv [Preprint]. 2024 Jun 29:2024.05.21.594886. doi: 10.1101/2024.05.21.594886. bioRxiv. 2024. PMID: 38979135 Free PMC article. Preprint.
-
A Mutant Variant of E2F4 Triggers Multifactorial Therapeutic Effects in 5xFAD Mice.Mol Neurobiol. 2022 May;59(5):3016-3039. doi: 10.1007/s12035-022-02764-z. Epub 2022 Mar 7. Mol Neurobiol. 2022. PMID: 35254651 Free PMC article.
-
Biological function of Lemur tyrosine kinase 2 (LMTK2): implications in neurodegeneration.Mol Brain. 2018 Apr 10;11(1):20. doi: 10.1186/s13041-018-0363-x. Mol Brain. 2018. PMID: 29631601 Free PMC article. Review.
References
-
- Fisher RP. The CDK network: linking cycles of cell division and gene expression. Genes Cancer 2012; 3:731-8; PMID:23634260; http://dx.doi.org/10.1177/1947601912473308 - DOI - PMC - PubMed
-
- Williams GH, Stoeber K. The cell cycle and cancer. J Pathol 2012; 226:352-64; PMID:21990031; http://dx.doi.org/10.1002/path.3022 - DOI - PubMed
-
- Hochegger H, Takeda S, Hunt T. Cyclin-dependent kinases and cell-cycle transitions: does one fit all? Nat Rev Mol Cell Biol 2008; 9:910-6; PMID:18813291; http://dx.doi.org/10.1038/nrm2510 - DOI - PubMed
-
- Liu N, Lucibello FC, Engeland K, Müller R. A new model of cell cycle-regulated transcription: repression of the cyclin A promoter by CDF-1 and anti-repression by E2F. Oncogene 1998; 16:2957-63; PMID:9662327 - PubMed
-
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 1999; 13:1501-12; PMID:10385618 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
