Of flies, mice, and men: evolutionarily conserved tissue damage responses and aging
- PMID: 25584795
- PMCID: PMC4450349
- DOI: 10.1016/j.devcel.2014.11.028
Of flies, mice, and men: evolutionarily conserved tissue damage responses and aging
Abstract
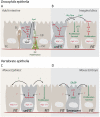
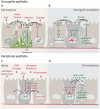
Studies in flies, mice, and human models have provided a conceptual framework for how paracrine interactions between damaged cells and the surrounding tissue control tissue repair. These studies have amassed evidence for an evolutionarily conserved secretory program that regulates tissue homeostasis. This program coordinates cell survival and proliferation during tissue regeneration and repair in young animals. By virtue of chronic engagement, however, it also contributes to the age-related decline of tissue homeostasis leading to degeneration, metabolic dysfunction, and cancer. Here, we review recent studies that shed light on the nature and regulation of this evolutionarily conserved secretory program.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Reparative inflammation takes charge of tissue regeneration.Nature. 2016 Jan 21;529(7586):307-15. doi: 10.1038/nature17039. Nature. 2016. PMID: 26791721 Free PMC article. Review.
-
Cell Autonomous and Non-Autonomous Effects of Senescent Cells in the Skin.J Invest Dermatol. 2015 Jul;135(7):1722-1726. doi: 10.1038/jid.2015.108. Epub 2015 Apr 9. J Invest Dermatol. 2015. PMID: 25855157 Free PMC article. Review.
-
Tipping the balance: modulating the Wnt pathway for tissue repair.Trends Biotechnol. 2009 Mar;27(3):131-6. doi: 10.1016/j.tibtech.2008.11.007. Epub 2009 Jan 31. Trends Biotechnol. 2009. PMID: 19187992 Review.
-
Effect of aging on wound healing: current concepts.J Wound Ostomy Continence Nurs. 2007 Jul-Aug;34(4):412-5; quiz 416-7. doi: 10.1097/01.WON.0000281658.71072.e6. J Wound Ostomy Continence Nurs. 2007. PMID: 17667088 Review.
-
MANF regulates metabolic and immune homeostasis in ageing and protects against liver damage.Nat Metab. 2019 Feb;1(2):276-290. doi: 10.1038/s42255-018-0023-6. Epub 2019 Jan 14. Nat Metab. 2019. PMID: 31489403 Free PMC article.
Cited by
-
Distinct signaling signatures drive compensatory proliferation via S-phase acceleration.PLoS Genet. 2022 Dec 15;18(12):e1010516. doi: 10.1371/journal.pgen.1010516. eCollection 2022 Dec. PLoS Genet. 2022. PMID: 36520882 Free PMC article.
-
Drosophila Gut-A Nexus Between Dietary Restriction and Lifespan.Int J Mol Sci. 2018 Nov 29;19(12):3810. doi: 10.3390/ijms19123810. Int J Mol Sci. 2018. PMID: 30501099 Free PMC article. Review.
-
Extracellular Vesicles and Damage-Associated Molecular Patterns: A Pandora's Box in Health and Disease.Front Immunol. 2020 Nov 16;11:601740. doi: 10.3389/fimmu.2020.601740. eCollection 2020. Front Immunol. 2020. PMID: 33304353 Free PMC article. Review.
-
Trophic Factors in Inflammation and Regeneration: The Role of MANF and CDNF.Front Physiol. 2018 Nov 20;9:1629. doi: 10.3389/fphys.2018.01629. eCollection 2018. Front Physiol. 2018. PMID: 30515104 Free PMC article.
-
A proteomic atlas of senescence-associated secretomes for aging biomarker development.PLoS Biol. 2020 Jan 16;18(1):e3000599. doi: 10.1371/journal.pbio.3000599. eCollection 2020 Jan. PLoS Biol. 2020. PMID: 31945054 Free PMC article.
References
-
- Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Furnagalli M, DaCosta M, Brown C, Popov N, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. - PubMed
-
- Adams PD. Healing and hurting: molecular mechanisms, functions and pathologies of cellular senescence. Molec Cell. 2009;36:2–14. - PubMed
-
- Adler PN, Bryant PJ. Participation of lethally irradiated imaginal disc tissue in pattern regulation in Drosophila. Developmental biology. 1977;60:298–304. - PubMed
-
- Agaisse H, Petersen UM, Boutros M, Mathey-Prevot B, Perrimon N. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Developmental cell. 2003;5:441–450. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 EY018177/EY/NEI NIH HHS/United States
- GM100196/GM/NIGMS NIH HHS/United States
- AG041122/AG/NIA NIH HHS/United States
- AG028127/AG/NIA NIH HHS/United States
- R01 AG028127/AG/NIA NIH HHS/United States
- AG009909/AG/NIA NIH HHS/United States
- AG017242/AG/NIA NIH HHS/United States
- R37 AG009909/AG/NIA NIH HHS/United States
- R56 AG009909/AG/NIA NIH HHS/United States
- P01 AG041122/AG/NIA NIH HHS/United States
- R01 GM100196/GM/NIGMS NIH HHS/United States
- P01 AG017242/AG/NIA NIH HHS/United States
- EY018177/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

