Native architecture of the Chlamydomonas chloroplast revealed by in situ cryo-electron tomography
- PMID: 25584625
- PMCID: PMC4292175
- DOI: 10.7554/eLife.04889
Native architecture of the Chlamydomonas chloroplast revealed by in situ cryo-electron tomography
Erratum in
-
Correction: Native architecture of the Chlamydomonas chloroplast revealed by in situ cryo-electron tomography.Elife. 2015 Sep 14;4:e11383. doi: 10.7554/eLife.11383. Elife. 2015. PMID: 26367339 Free PMC article. No abstract available.
Abstract
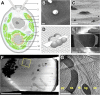
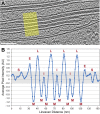
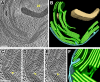
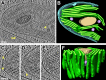
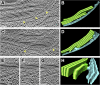
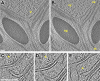
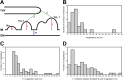
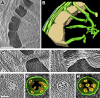
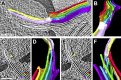
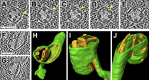
Chloroplast function is orchestrated by the organelle's intricate architecture. By combining cryo-focused ion beam milling of vitreous Chlamydomonas cells with cryo-electron tomography, we acquired three-dimensional structures of the chloroplast in its native state within the cell. Chloroplast envelope inner membrane invaginations were frequently found in close association with thylakoid tips, and the tips of multiple thylakoid stacks converged at dynamic sites on the chloroplast envelope, implicating lipid transport in thylakoid biogenesis. Subtomogram averaging and nearest neighbor analysis revealed that RuBisCO complexes were hexagonally packed within the pyrenoid, with ~15 nm between their centers. Thylakoid stacks and the pyrenoid were connected by cylindrical pyrenoid tubules, physically bridging the sites of light-dependent photosynthesis and light-independent carbon fixation. Multiple parallel minitubules were bundled within each pyrenoid tubule, possibly serving as conduits for the targeted one-dimensional diffusion of small molecules such as ATP and sugars between the chloroplast stroma and the pyrenoid matrix.
Keywords: Chlamydomonas; RuBisCO; biophysics; chloroplast; cryo-electron tomography; focused ion beam; plant biology; structural biology; thylakoid.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures


Similar articles
-
Charting the native architecture of Chlamydomonas thylakoid membranes with single-molecule precision.Elife. 2020 Apr 16;9:e53740. doi: 10.7554/eLife.53740. Elife. 2020. PMID: 32297859 Free PMC article.
-
The internal plumbing of algal chloroplasts.Elife. 2015 Jan 13;4:e05983. doi: 10.7554/eLife.05983. Elife. 2015. PMID: 25584626 Free PMC article.
-
Pyrenoid loss in Chlamydomonas reinhardtii causes limitations in CO2 supply, but not thylakoid operating efficiency.J Exp Bot. 2017 Jun 1;68(14):3903-3913. doi: 10.1093/jxb/erx197. J Exp Bot. 2017. PMID: 28911055 Free PMC article.
-
The algal pyrenoid: key unanswered questions.J Exp Bot. 2017 Jun 1;68(14):3739-3749. doi: 10.1093/jxb/erx178. J Exp Bot. 2017. PMID: 28911054 Review.
-
Electron tomography of plant thylakoid membranes.J Exp Bot. 2011 Apr;62(7):2393-402. doi: 10.1093/jxb/err034. Epub 2011 Mar 25. J Exp Bot. 2011. PMID: 21441405 Review.
Cited by
-
Isolation and Expression Analysis of Three Types of α-Carbonic Anhydrases from the Antarctic Alga Chlamydomonas sp. ICE-L under Different Light Stress Treatments.Mol Biotechnol. 2019 Mar;61(3):200-208. doi: 10.1007/s12033-018-00152-4. Mol Biotechnol. 2019. PMID: 30649663
-
Chlororespiration Controls Growth Under Intermittent Light.Plant Physiol. 2019 Feb;179(2):630-639. doi: 10.1104/pp.18.01213. Epub 2018 Nov 29. Plant Physiol. 2019. PMID: 30498023 Free PMC article.
-
A Series of Fortunate Events: Introducing Chlamydomonas as a Reference Organism.Plant Cell. 2019 Aug;31(8):1682-1707. doi: 10.1105/tpc.18.00952. Epub 2019 Jun 12. Plant Cell. 2019. PMID: 31189738 Free PMC article. Review.
-
Preparing Arabidopsis thaliana root protoplasts for cryo electron tomography.Front Plant Sci. 2023 Sep 22;14:1261180. doi: 10.3389/fpls.2023.1261180. eCollection 2023. Front Plant Sci. 2023. PMID: 37810374 Free PMC article.
-
Structural Basis of Vesicle Formation at the Inner Nuclear Membrane.Cell. 2015 Dec 17;163(7):1692-701. doi: 10.1016/j.cell.2015.11.029. Cell. 2015. PMID: 26687357 Free PMC article.
References
-
- Armbruster U, Labs M, Pribil M, Viola S, Xu W, Scharfenberg M, Hertle AP, Rojahn U, Jensen PE, Rappaport F, Joliot P, Dörmann P, Wanner G, Leister D. Arabidopsis CURVATURE THYLAKOID1 proteins modify thylakoid architecture by inducing membrane curvature. The Plant Cell. 2013;25:2661–2678. doi: 10.1105/tpc.113.113118. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

