Microbiota controls the homeostasis of glial cells in the gut lamina propria
- PMID: 25578362
- PMCID: PMC4306542
- DOI: 10.1016/j.neuron.2014.12.037
Microbiota controls the homeostasis of glial cells in the gut lamina propria
Abstract
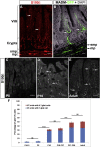
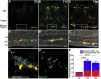
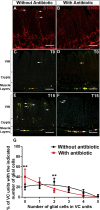
The intrinsic neural networks of the gastrointestinal tract are derived from dedicated neural crest progenitors that colonize the gut during embryogenesis and give rise to enteric neurons and glia. Here, we study how an essential subpopulation of enteric glial cells (EGCs) residing within the intestinal mucosa is integrated into the dynamic microenvironment of the alimentary tract. We find that under normal conditions colonization of the lamina propria by glial cells commences during early postnatal stages but reaches steady-state levels after weaning. By employing genetic lineage tracing, we provide evidence that in adult mice the network of mucosal EGCs is continuously renewed by incoming glial cells originating in the plexi of the gut wall. Finally, we demonstrate that both the initial colonization and homeostasis of glial cells in the intestinal mucosa are regulated by the indigenous gut microbiota.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

Comment in
-
Bugs, guts, and glia: how microbiota influence enteric gliogenesis and migration.Neuron. 2015 Jan 21;85(2):229-30. doi: 10.1016/j.neuron.2014.12.066. Neuron. 2015. PMID: 25611502 Free PMC article.
Similar articles
-
Bugs, guts, and glia: how microbiota influence enteric gliogenesis and migration.Neuron. 2015 Jan 21;85(2):229-30. doi: 10.1016/j.neuron.2014.12.066. Neuron. 2015. PMID: 25611502 Free PMC article.
-
Isolation of Enteric Glial Cells from the Submucosa and Lamina Propria of the Adult Mouse.J Vis Exp. 2018 Aug 15;(138):57629. doi: 10.3791/57629. J Vis Exp. 2018. PMID: 30175991 Free PMC article.
-
Homeostasis of mucosal glial cells in human gut is independent of microbiota.Sci Rep. 2021 Jun 17;11(1):12796. doi: 10.1038/s41598-021-92384-9. Sci Rep. 2021. PMID: 34140608 Free PMC article.
-
From diversity to disease: unravelling the role of enteric glial cells.Front Immunol. 2024 Jun 18;15:1408744. doi: 10.3389/fimmu.2024.1408744. eCollection 2024. Front Immunol. 2024. PMID: 38957473 Free PMC article. Review.
-
Novel functional roles for enteric glia in the gastrointestinal tract.Nat Rev Gastroenterol Hepatol. 2012 Nov;9(11):625-32. doi: 10.1038/nrgastro.2012.138. Epub 2012 Aug 14. Nat Rev Gastroenterol Hepatol. 2012. PMID: 22890111 Review.
Cited by
-
Enteric glia as a source of neural progenitors in adult zebrafish.Elife. 2020 Aug 27;9:e56086. doi: 10.7554/eLife.56086. Elife. 2020. PMID: 32851974 Free PMC article.
-
Changes in Nicotinic Neurotransmission during Enteric Nervous System Development.J Neurosci. 2015 May 6;35(18):7106-15. doi: 10.1523/JNEUROSCI.4175-14.2015. J Neurosci. 2015. PMID: 25948261 Free PMC article.
-
Nerves in gastrointestinal cancer: from mechanism to modulations.Nat Rev Gastroenterol Hepatol. 2022 Dec;19(12):768-784. doi: 10.1038/s41575-022-00669-9. Epub 2022 Sep 2. Nat Rev Gastroenterol Hepatol. 2022. PMID: 36056202 Review.
-
Intestinal Permeability, Dysbiosis, Inflammation and Enteric Glia Cells: The Intestinal Etiology of Parkinson's Disease.Aging Dis. 2022 Oct 1;13(5):1381-1390. doi: 10.14336/AD.2022.01281. eCollection 2022 Oct 1. Aging Dis. 2022. PMID: 36186124 Free PMC article.
-
Gastrointestinal hormones and the gut connectome.Curr Opin Endocrinol Diabetes Obes. 2017 Feb;24(1):9-14. doi: 10.1097/MED.0000000000000299. Curr Opin Endocrinol Diabetes Obes. 2017. PMID: 27820704 Free PMC article. Review.
References
-
- Abrams G.D., Bishop J.E. Effect of the normal microbial flora on gastrointestinal motility. Proc. Soc. Exp. Biol. Med. 1967;126:301–304. - PubMed
-
- Boesmans W., Lasrado R., Vanden Berghe P., Pachnis V. Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia. 2015;63:229–241. Published online August 26, 2014. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

