Syndecan-4 is a major syndecan in primary human endothelial cells in vitro, modulated by inflammatory stimuli and involved in wound healing
- PMID: 25575567
- PMCID: PMC4374057
- DOI: 10.1369/0022155415568995
Syndecan-4 is a major syndecan in primary human endothelial cells in vitro, modulated by inflammatory stimuli and involved in wound healing
Abstract
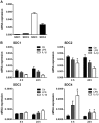
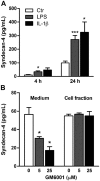
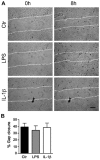
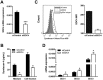
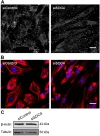
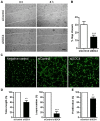
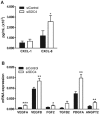
Syndecans are important cell surface proteoglycans with many functions; yet, they have not been studied to a very large extent in primary human endothelial cells. The purpose of this study was to investigate syndecan-4 expression in cultured human umbilical vein endothelial cells (HUVECs) and assess its role in inflammatory reactions and experimental wound healing. qRT-PCR analysis revealed that syndecan-3 and syndecan-4 were highly expressed in HUVECs, whereas the expression of syndecan-1 and -2 was low. HUVECs were cultured with the inflammatory mediators lipopolysaccharide (LPS) and interleukin 1β (IL-1β). As a result, syndecan-4 expression showed a rapid and strong increase. Syndecan-1 and -2 expressions decreased, whereas syndecan-3 was unaffected. Knockdown of syndecan-4 using siRNA resulted in changes in cellular morphology and focal adhesion sites, delayed wound healing and tube formation, and increased secretion of the pro-inflammatory and angiogenic chemokine, CXCL8. These data suggest functions for syndecan-4 in inflammatory reactions, wound healing and angiogenesis in primary human endothelial cells.
Keywords: angiogenesis; inflammation; primary human endothelial cells; shedding; syndecan-4; wound healing.
© The Author(s) 2015.
Conflict of interest statement
Figures


Similar articles
-
Serglycin secretion is part of the inflammatory response in activated primary human endothelial cells in vitro.Biochim Biophys Acta. 2014 Aug;1840(8):2498-505. doi: 10.1016/j.bbagen.2014.02.002. Epub 2014 Feb 7. Biochim Biophys Acta. 2014. PMID: 24513305
-
Innate immune signaling induces expression and shedding of the heparan sulfate proteoglycan syndecan-4 in cardiac fibroblasts and myocytes, affecting inflammation in the pressure-overloaded heart.FEBS J. 2013 May;280(10):2228-47. doi: 10.1111/febs.12161. Epub 2013 Feb 24. FEBS J. 2013. PMID: 23374111
-
Expression of heparan sulfate proteoglycans in murine models of experimental colitis.Inflamm Bowel Dis. 2012 Jun;18(6):1112-26. doi: 10.1002/ibd.21879. Epub 2011 Oct 10. Inflamm Bowel Dis. 2012. PMID: 21987406
-
Soluble vascular endothelial glycocalyx proteoglycans as potential therapeutic targets in inflammatory diseases.Immunol Cell Biol. 2024 Feb;102(2):97-116. doi: 10.1111/imcb.12712. Epub 2023 Nov 20. Immunol Cell Biol. 2024. PMID: 37982607 Review.
-
Thy-1 (CD90), Integrins and Syndecan 4 are Key Regulators of Skin Wound Healing.Front Cell Dev Biol. 2022 Feb 3;10:810474. doi: 10.3389/fcell.2022.810474. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35186924 Free PMC article. Review.
Cited by
-
Syndecan 4 is a marker of endothelial inflammation in pathological aging and predicts long-term cardiovascular outcomes in type 2 diabetes.Diabetol Metab Syndr. 2024 Aug 20;16(1):203. doi: 10.1186/s13098-024-01431-8. Diabetol Metab Syndr. 2024. PMID: 39164788 Free PMC article.
-
Association between syndecan-4 and subclinical atherosclerosis in ankylosing spondylitis.Medicine (Baltimore). 2024 Jan 19;103(3):e37019. doi: 10.1097/MD.0000000000037019. Medicine (Baltimore). 2024. PMID: 38241528 Free PMC article.
-
Capillary leak and endothelial permeability in critically ill patients: a current overview.Intensive Care Med Exp. 2023 Dec 20;11(1):96. doi: 10.1186/s40635-023-00582-8. Intensive Care Med Exp. 2023. PMID: 38117435 Free PMC article. Review.
-
Thy-1 (CD90)-regulated cell adhesion and migration of mesenchymal cells: insights into adhesomes, mechanical forces, and signaling pathways.Front Cell Dev Biol. 2023 Nov 30;11:1221306. doi: 10.3389/fcell.2023.1221306. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38099295 Free PMC article. Review.
-
Effects of Heparan sulfate acetyl-CoA: Alpha-glucosaminide N-acetyltransferase (HGSNAT) inactivation on the structure and function of epithelial and immune cells of the testis and epididymis and sperm parameters in adult mice.PLoS One. 2023 Sep 27;18(9):e0292157. doi: 10.1371/journal.pone.0292157. eCollection 2023. PLoS One. 2023. PMID: 37756356 Free PMC article.
References
-
- Alexopoulou AN, Multhaupt HA, Couchman JR. (2007). Syndecans in wound healing, inflammation and vascular biology. Int J Biochem Cell Biol 39:505-28. - PubMed
-
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. (1999). Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem 68:729-77. - PubMed
-
- Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, Lose EJ. (1992). Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol 8:365-93. - PubMed
-
- Chalkiadaki G, Nikitovic D, Berdiaki A, Sifaki M, Krasagakis K, Katonis P, Karamanos NK, Tzanakakis GN. (2009). Fibroblast growth factor-2 modulates melanoma adhesion and migration through a syndecan-4-dependent mechanism. Int J Biochem Cell Biol 41:1323-1331. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

