p73 is required for endothelial cell differentiation, migration and the formation of vascular networks regulating VEGF and TGFβ signaling
- PMID: 25571973
- PMCID: PMC4495354
- DOI: 10.1038/cdd.2014.214
p73 is required for endothelial cell differentiation, migration and the formation of vascular networks regulating VEGF and TGFβ signaling
Abstract
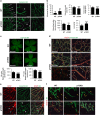
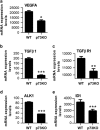
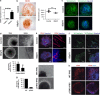
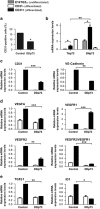
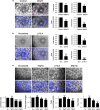
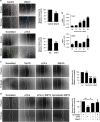
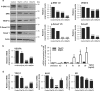
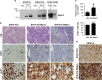
Vasculogenesis, the establishment of the vascular plexus and angiogenesis, branching of new vessels from the preexisting vasculature, involves coordinated endothelial differentiation, proliferation and migration. Disturbances in these coordinated processes may accompany diseases such as cancer. We hypothesized that the p53 family member p73, which regulates cell differentiation in several contexts, may be important in vascular development. We demonstrate that p73 deficiency perturbed vascular development in the mouse retina, decreasing vascular branching, density and stability. Furthermore, p73 deficiency could affect non endothelial cells (ECs) resulting in reduced in vivo proangiogenic milieu. Moreover, p73 functional inhibition, as well as p73 deficiency, hindered vessel sprouting, tubulogenesis and the assembly of vascular structures in mouse embryonic stem cell and induced pluripotent stem cell cultures. Therefore, p73 is necessary for EC biology and vasculogenesis and, in particular, that DNp73 regulates EC migration and tube formation capacity by regulation of expression of pro-angiogenic factors such as transforming growth factor-β and vascular endothelial growth factors. DNp73 expression is upregulated in the tumor environment, resulting in enhanced angiogenic potential of B16-F10 melanoma cells. Our results demonstrate, by the first time, that differential p73-isoform regulation is necessary for physiological vasculogenesis and angiogenesis and DNp73 overexpression becomes a positive advantage for tumor progression due to its pro-angiogenic capacity.
Figures
Similar articles
-
Hypoxia-induced DNp73 stabilization regulates Vegf-A expression and tumor angiogenesis similar to TAp73.Cell Cycle. 2015;14(22):3533-9. doi: 10.1080/15384101.2015.1078038. Cell Cycle. 2015. PMID: 26267146 Free PMC article.
-
miR-342-5p Is a Notch Downstream Molecule and Regulates Multiple Angiogenic Pathways Including Notch, Vascular Endothelial Growth Factor and Transforming Growth Factor β Signaling.J Am Heart Assoc. 2016 Feb 8;5(2):e003042. doi: 10.1161/JAHA.115.003042. J Am Heart Assoc. 2016. PMID: 26857067 Free PMC article.
-
DNp73 improves generation efficiency of human induced pluripotent stem cells.BMC Cell Biol. 2012 Mar 26;13:9. doi: 10.1186/1471-2121-13-9. BMC Cell Biol. 2012. PMID: 22449255 Free PMC article.
-
Mechanisms, function and clinical applications of DNp73.Cell Cycle. 2013 Jun 15;12(12):1861-7. doi: 10.4161/cc.24967. Epub 2013 Jun 13. Cell Cycle. 2013. PMID: 23708520 Free PMC article. Review.
-
p73: a Positive or Negative Regulator of Angiogenesis, or Both?Mol Cell Biol. 2015 Dec 28;36(6):848-54. doi: 10.1128/MCB.00929-15. Mol Cell Biol. 2015. PMID: 26711266 Free PMC article. Review.
Cited by
-
p53 Family in Resistance to Targeted Therapy of Melanoma.Int J Mol Sci. 2022 Dec 21;24(1):65. doi: 10.3390/ijms24010065. Int J Mol Sci. 2022. PMID: 36613518 Free PMC article. Review.
-
Mesenchymal Stem Cells Differentiate to Endothelial Cells Using Recombinant Vascular Endothelial Growth Factor -A.Rep Biochem Mol Biol. 2018 Apr;6(2):144-150. Rep Biochem Mol Biol. 2018. PMID: 29761109 Free PMC article.
-
The hypoxic tumour microenvironment.Oncogenesis. 2018 Jan 24;7(1):10. doi: 10.1038/s41389-017-0011-9. Oncogenesis. 2018. PMID: 29362402 Free PMC article. Review.
-
ΔNp73 enhances HIF-1α protein stability through repression of the ECV complex.Oncogene. 2018 Jul;37(27):3729-3739. doi: 10.1038/s41388-018-0195-2. Epub 2018 Apr 9. Oncogene. 2018. PMID: 29628507 Free PMC article.
-
p73 as a Tissue Architect.Front Cell Dev Biol. 2021 Jul 23;9:716957. doi: 10.3389/fcell.2021.716957. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34368167 Free PMC article. Review.
References
-
- Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. - PubMed
-
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. - PubMed
-
- Blagosklonny MV. Apoptosis, proliferation, differentiation: in search of the order. Semin Cancer Biol. 2003;13:97–105. - PubMed
-
- Salimath B, Marme D, Finkenzeller G. Expression of the vascular endothelial growth factor gene is inhibited by p73. Oncogene. 2000;19:3470–3476. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

