Light-responsive nanoparticle depot to control release of a small molecule angiogenesis inhibitor in the posterior segment of the eye
- PMID: 25571784
- PMCID: PMC4384820
- DOI: 10.1016/j.jconrel.2015.01.001
Light-responsive nanoparticle depot to control release of a small molecule angiogenesis inhibitor in the posterior segment of the eye
Erratum in
- J Control Release. 2015 Apr 10;203:39
Abstract
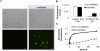
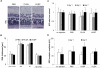
Therapies for macular degeneration and diabetic retinopathy require intravitreal injections every 4-8 weeks. Injections are uncomfortable, time-consuming, and carry risks of infection and retinal damage. However, drug delivery via noninvasive methods to the posterior segment of the eye has been a major challenge due to the eye's unique anatomy and physiology. Here we present a novel nanoparticle depot platform for on-demand drug delivery using a far ultraviolet (UV) light-degradable polymer, which allows noninvasively triggered drug release using brief, low-power light exposure. Nanoparticles stably retain encapsulated molecules in the vitreous, and can release cargo in response to UV exposure up to 30 weeks post-injection. Light-triggered release of nintedanib (BIBF 1120), a small molecule angiogenesis inhibitor, 10 weeks post-injection suppresses choroidal neovascularization (CNV) in rats. Light-sensitive nanoparticles are biocompatible and cause no adverse effects on the eye as assessed by electroretinograms (ERG), corneal and retinal tomography, and histology.
Keywords: Anti-angiogenic; Light-triggered; Nanoparticle; Ocular; Polymer; Triggered release.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Long-acting nanoparticle-loaded bilayer microneedles for protein delivery to the posterior segment of the eye.Eur J Pharm Biopharm. 2021 Aug;165:306-318. doi: 10.1016/j.ejpb.2021.05.022. Epub 2021 May 26. Eur J Pharm Biopharm. 2021. PMID: 34048879
-
Fenofibrate-Loaded Biodegradable Nanoparticles for the Treatment of Experimental Diabetic Retinopathy and Neovascular Age-Related Macular Degeneration.Mol Pharm. 2019 May 6;16(5):1958-1970. doi: 10.1021/acs.molpharmaceut.8b01319. Epub 2019 Mar 26. Mol Pharm. 2019. PMID: 30912953 Free PMC article.
-
Anti-inflammatory and antiangiogenic effects of nanoparticle-mediated delivery of a natural angiogenic inhibitor.Invest Ophthalmol Vis Sci. 2011 Aug 5;52(9):6230-7. doi: 10.1167/iovs.10-6229. Invest Ophthalmol Vis Sci. 2011. PMID: 21357401 Free PMC article.
-
Biodegradable Microspheres as Intravitreal Delivery Systems for Prolonged Drug Release. What is their Eminence in the Nanoparticle Era?Curr Drug Deliv. 2018;15(7):930-940. doi: 10.2174/1567201815666180226121020. Curr Drug Deliv. 2018. PMID: 29484995 Review.
-
Nanoparticles for the treatment of ocular neovascularizations.Eur J Pharm Biopharm. 2015 Sep;95(Pt B):294-306. doi: 10.1016/j.ejpb.2015.02.027. Epub 2015 Mar 7. Eur J Pharm Biopharm. 2015. PMID: 25758124 Review.
Cited by
-
Ten Years of Knowledge of Nano-Carrier Based Drug Delivery Systems in Ophthalmology: Current Evidence, Challenges, and Future Prospective.Int J Nanomedicine. 2021 Sep 22;16:6497-6530. doi: 10.2147/IJN.S329831. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34588777 Free PMC article. Review.
-
Optically controlled release of DNA based on nonradiative relaxation process of quenchers.Biomed Opt Express. 2016 May 9;7(6):2142-53. doi: 10.1364/BOE.7.002142. eCollection 2016 Jun 1. Biomed Opt Express. 2016. PMID: 27375933 Free PMC article.
-
Ocular Nanomedicine.Adv Sci (Weinh). 2022 May;9(15):e2003699. doi: 10.1002/advs.202003699. Epub 2022 Feb 12. Adv Sci (Weinh). 2022. PMID: 35150092 Free PMC article. Review.
-
Light-responsive biomaterials for ocular drug delivery.Drug Deliv Transl Res. 2023 Aug;13(8):2159-2182. doi: 10.1007/s13346-022-01196-5. Epub 2022 Jun 24. Drug Deliv Transl Res. 2023. PMID: 35751001 Free PMC article. Review.
-
Stem Cell Imaging: Tools to Improve Cell Delivery and Viability.Stem Cells Int. 2016;2016:9240652. doi: 10.1155/2016/9240652. Epub 2016 Jan 6. Stem Cells Int. 2016. PMID: 26880997 Free PMC article. Review.
References
-
- Zhang K, Zhang L, Weinreb RN. Ophthalmic drug discovery: novel targets and mechanisms for retinal diseases and glaucoma. Nat Rev Drug Discov. 2012;11:541–559. - PubMed
-
- Nguyen DH, Luo J, Zhang K, Zhang M. Current therapeutic approaches in neovascular age-related macular degeneration. Discov Med. 2013;15:343–348. - PubMed
-
- Herrero-Vanrell R, Refojo MF. Biodegradable microspheres for vitreoretinal drug delivery. Adv Drug Deliv Rev. 2001;52:5–16. - PubMed
-
- Bourges JL, Bloquel C, Thomas A, Froussart F, Bochot A, Azan F, Gurny R, BenEzra D, Behar-Cohen F. Intraocular implants for extended drug delivery: therapeutic applications. Adv Drug Deliv Rev. 2006;58:1182–1202. - PubMed
-
- Ali Y, Lehmussaari K. Industrial perspective in ocular drug delivery. Adv Drug Deliv Rev. 2006;58:1258–1268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

