Advances in anticancer immunotoxin therapy
- PMID: 25561510
- PMCID: PMC4319635
- DOI: 10.1634/theoncologist.2014-0358
Advances in anticancer immunotoxin therapy
Abstract
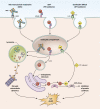
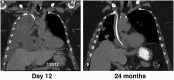
Immunotoxins are a novel class of antibody-conjugated therapeutics currently in clinical development for a variety of malignancies. They consist of an antibody-based targeting domain fused to a bacterial toxin payload for cell killing. Immunotoxins kill cells by inhibiting protein synthesis, a unique mechanism of action that is toxic to both dividing and nondividing cells. Recent advances in the design and administration of immunotoxins are overcoming historical challenges in the field, leading to renewed interest in these therapeutics.
Keywords: Antibody conjugate; Antidrug antibody; Recombinant immunotoxin; Vascular leak syndrome.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures

Similar articles
-
Recombinant antibody fragments and immunotoxin fusions for cancer therapy.In Vivo. 2000 Jan-Feb;14(1):21-7. In Vivo. 2000. PMID: 10757057 Review.
-
Anti-cancer Immunotoxins, Challenges, and Approaches.Curr Pharm Des. 2021;27(7):932-941. doi: 10.2174/1381612826666201006155346. Curr Pharm Des. 2021. PMID: 33023437
-
Recombinant immunotoxins containing truncated bacterial toxins for the treatment of hematologic malignancies.BioDrugs. 2009;23(1):1-13. doi: 10.2165/00063030-200923010-00001. BioDrugs. 2009. PMID: 19344187 Free PMC article. Review.
-
Immunotoxin therapy of cancer.Nat Rev Cancer. 2006 Jul;6(7):559-65. doi: 10.1038/nrc1891. Nat Rev Cancer. 2006. PMID: 16794638 Review.
-
Humanized immunotoxins: a new generation of immunotoxins for targeted cancer therapy.Cancer Sci. 2009 Aug;100(8):1359-65. doi: 10.1111/j.1349-7006.2009.01192.x. Epub 2009 May 19. Cancer Sci. 2009. PMID: 19459847 Free PMC article. Review.
Cited by
-
Genetically modified proteins: functional improvement and chimeragenesis.Bioengineered. 2015;6(5):262-74. doi: 10.1080/21655979.2015.1075674. Epub 2015 Jul 25. Bioengineered. 2015. PMID: 26211369 Free PMC article. Review.
-
Nanobody-Based EGFR-Targeting Immunotoxins for Colorectal Cancer Treatment.Biomolecules. 2023 Jun 26;13(7):1042. doi: 10.3390/biom13071042. Biomolecules. 2023. PMID: 37509078 Free PMC article.
-
Immunotoxin SS1P is rapidly removed by proximal tubule cells of kidney, whose damage contributes to albumin loss in urine.Proc Natl Acad Sci U S A. 2020 Mar 17;117(11):6086-6091. doi: 10.1073/pnas.1919038117. Epub 2020 Mar 2. Proc Natl Acad Sci U S A. 2020. PMID: 32123080 Free PMC article.
-
Halfway between 2D and Animal Models: Are 3D Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions?Int J Mol Sci. 2018 Jan 18;19(1):181. doi: 10.3390/ijms19010181. Int J Mol Sci. 2018. PMID: 29346265 Free PMC article. Review.
-
Immunotoxin Therapy for Lung Cancer.Chin Med J (Engl). 2017 Mar 5;130(5):607-612. doi: 10.4103/0366-6999.200540. Chin Med J (Engl). 2017. PMID: 28229994 Free PMC article. No abstract available.
References
-
- Collier RJ. Effect of diphtheria toxin on protein synthesis: Inactivation of one of the transfer factors. J Mol Biol. 1967;25:83–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

