Global analysis of myocardial peptides containing cysteines with irreversible sulfinic and sulfonic acid post-translational modifications
- PMID: 25561502
- PMCID: PMC4349981
- DOI: 10.1074/mcp.M114.044347
Global analysis of myocardial peptides containing cysteines with irreversible sulfinic and sulfonic acid post-translational modifications
Abstract
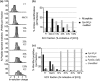
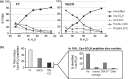
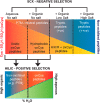
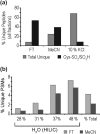
Cysteine (Cys) oxidation is a crucial post-translational modification (PTM) associated with redox signaling and oxidative stress. As Cys is highly reactive to oxidants it forms a range of post-translational modifications, some that are biologically reversible (e.g. disulfides, Cys sulfenic acid) and others (Cys sulfinic [Cys-SO2H] and sulfonic [Cys-SO3H] acids) that are considered "irreversible." We developed an enrichment method to isolate Cys-SO2H/SO3H-containing peptides from complex tissue lysates that is compatible with tandem mass spectrometry (MS/MS). The acidity of these post-translational modification (pKa Cys-SO3H < 0) creates a unique charge distribution when localized on tryptic peptides at acidic pH that can be utilized for their purification. The method is based on electrostatic repulsion of Cys-SO2H/SO3H-containing peptides from cationic resins (i.e. "negative" selection) followed by "positive" selection using hydrophilic interaction liquid chromatography. Modification of strong cation exchange protocols decreased the complexity of initial flowthrough fractions by allowing for hydrophobic retention of neutral peptides. Coupling of strong cation exchange and hydrophilic interaction liquid chromatography allowed for increased enrichment of Cys-SO2H/SO3H (up to 80%) from other modified peptides. We identified 181 Cys-SO2H/SO3H sites from rat myocardial tissue subjected to physiologically relevant concentrations of H2O2 (<100 μm) or to ischemia/reperfusion (I/R) injury via Langendorff perfusion. I/R significantly increased Cys-SO2H/SO3H-modified peptides from proteins involved in energy utilization and contractility, as well as those involved in oxidative damage and repair.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Similar articles
-
A Global Profile of Reversible and Irreversible Cysteine Redox Post-Translational Modifications During Myocardial Ischemia/Reperfusion Injury and Antioxidant Intervention.Antioxid Redox Signal. 2021 Jan 1;34(1):11-31. doi: 10.1089/ars.2019.7765. Epub 2020 Sep 25. Antioxid Redox Signal. 2021. PMID: 32729339
-
Large-scale capture of peptides containing reversibly oxidized cysteines by thiol-disulfide exchange applied to the myocardial redox proteome.Anal Chem. 2013 Apr 2;85(7):3774-80. doi: 10.1021/ac400166e. Epub 2013 Mar 12. Anal Chem. 2013. PMID: 23438843
-
Synthesis and conformational preferences of peptides and proteins with cysteine sulfonic acid.Org Biomol Chem. 2023 Mar 29;21(13):2779-2800. doi: 10.1039/d3ob00179b. Org Biomol Chem. 2023. PMID: 36920119
-
Cysteine thiol sulfinic acid in plant stress signaling.Plant Cell Environ. 2024 Aug;47(8):2766-2779. doi: 10.1111/pce.14827. Epub 2024 Jan 22. Plant Cell Environ. 2024. PMID: 38251793 Review.
-
Stabilising cysteinyl thiol oxidation and nitrosation for proteomic analysis.J Proteomics. 2013 Oct 30;92:160-70. doi: 10.1016/j.jprot.2013.06.019. Epub 2013 Jun 21. J Proteomics. 2013. PMID: 23796488 Review.
Cited by
-
Proteomics of the heart.Physiol Rev. 2024 Jul 1;104(3):931-982. doi: 10.1152/physrev.00026.2023. Epub 2024 Feb 1. Physiol Rev. 2024. PMID: 38300522 Free PMC article. Review.
-
Irreversible oxidative post-translational modifications in heart disease.Expert Rev Proteomics. 2019 Aug;16(8):681-693. doi: 10.1080/14789450.2019.1645602. Epub 2019 Jul 30. Expert Rev Proteomics. 2019. PMID: 31361162 Free PMC article. Review.
-
Redox Signaling by Reactive Electrophiles and Oxidants.Chem Rev. 2018 Sep 26;118(18):8798-8888. doi: 10.1021/acs.chemrev.7b00698. Epub 2018 Aug 27. Chem Rev. 2018. PMID: 30148624 Free PMC article. Review.
-
Redox regulation of cell proliferation: Bioinformatics and redox proteomics approaches to identify redox-sensitive cell cycle regulators.Free Radic Biol Med. 2018 Jul;122:137-149. doi: 10.1016/j.freeradbiomed.2018.03.047. Epub 2018 Mar 29. Free Radic Biol Med. 2018. PMID: 29605447 Free PMC article. Review.
-
ProtView: A Versatile Tool for In Silico Protease Evaluation and Selection in a Proteomic and Proteogenomic Context.J Proteome Res. 2023 Jul 7;22(7):2400-2410. doi: 10.1021/acs.jproteome.3c00135. Epub 2023 May 29. J Proteome Res. 2023. PMID: 37248202 Free PMC article.
References
-
- Dröge W. (2002) Free radicals in the physiological control of cell function. Physiol. Rev. 82, 47–95 - PubMed
-
- Valko M., Leibfritz D., Moncol J., Cronin M. T. D., Mazur M., Telser J. (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 39, 44–84 - PubMed
-
- Gygi S. P., Rist B., Gerber S. A., Turecek F., Gelb M. H., Aebersold R. (1999) Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat. Biotechnol. 17, 994–999 - PubMed
-
- Hägglund P., Bunkenborg J., Maeda K., Svensson B. (2008) Identification of thioredoxin disulfide targets using a quantitative proteomics approach based on isotope-coded affinity tags. J. Proteome Res. 7, 5270–5276 - PubMed
-
- Sethuraman M., McComb M. E., Heibeck T., Costello C. E., Cohen R. A. (2004) Isotope-coded affinity tag approach to identify and quantify oxidant-sensitive protein thiols. Mol. Cell. Proteomics 3, 273–278 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

