A laminin 511 matrix is regulated by TAZ and functions as the ligand for the α6Bβ1 integrin to sustain breast cancer stem cells
- PMID: 25561492
- PMCID: PMC4281560
- DOI: 10.1101/gad.253682.114
A laminin 511 matrix is regulated by TAZ and functions as the ligand for the α6Bβ1 integrin to sustain breast cancer stem cells
Abstract
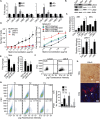
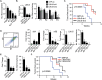
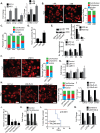
Understanding how the extracellular matrix impacts the function of cancer stem cells (CSCs) is a significant but poorly understood problem. We report that breast CSCs produce a laminin (LM) 511 matrix that promotes self-renewal and tumor initiation by engaging the α6Bβ1 integrin and activating the Hippo transducer TAZ. Although TAZ is important for the function of breast CSCs, the mechanism is unknown. We observed that TAZ regulates the transcription of the α5 subunit of LM511 and the formation of a LM511 matrix. These data establish a positive feedback loop involving TAZ and LM511 that contributes to stemness in breast cancer.
Keywords: TAZ; cancer stem cell; extracellular matrix; integrin; laminin.
© 2015 Chang et al.; Published by Cold Spring Harbor Laboratory Press.
Figures

Similar articles
-
VEGF-neuropilin-2 signaling promotes stem-like traits in breast cancer cells by TAZ-mediated repression of the Rac GAP β2-chimaerin.Sci Signal. 2018 May 1;11(528):eaao6897. doi: 10.1126/scisignal.aao6897. Sci Signal. 2018. PMID: 29717062 Free PMC article.
-
The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells.Cell. 2011 Nov 11;147(4):759-72. doi: 10.1016/j.cell.2011.09.048. Cell. 2011. PMID: 22078877
-
Axolotl pronephric duct migration requires an epidermally derived, laminin 1-containing extracellular matrix and the integrin receptor alpha6beta1.Development. 2003 Dec;130(23):5601-8. doi: 10.1242/dev.00765. Epub 2003 Oct 1. Development. 2003. PMID: 14522870
-
The laminin binding integrin alpha6beta1 in prostate cancer perineural invasion.J Cell Physiol. 2010 Aug;224(2):283-8. doi: 10.1002/jcp.22149. J Cell Physiol. 2010. PMID: 20432448 Free PMC article. Review.
-
Laminin-511: a multi-functional adhesion protein regulating cell migration, tumor invasion and metastasis.Cell Adh Migr. 2013 Jan-Feb;7(1):142-9. doi: 10.4161/cam.22125. Epub 2012 Oct 17. Cell Adh Migr. 2013. PMID: 23076212 Free PMC article. Review.
Cited by
-
Physiological mechanisms and therapeutic potential of bone mechanosensing.Rev Endocr Metab Disord. 2015 Jun;16(2):115-29. doi: 10.1007/s11154-015-9313-4. Rev Endocr Metab Disord. 2015. PMID: 26038304 Free PMC article. Review.
-
The Role of the Extracellular Matrix in Cancer Stemness.Front Cell Dev Biol. 2019 Jul 5;7:86. doi: 10.3389/fcell.2019.00086. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31334229 Free PMC article.
-
YAP/TAZ upstream signals and downstream responses.Nat Cell Biol. 2018 Aug;20(8):888-899. doi: 10.1038/s41556-018-0142-z. Epub 2018 Jul 26. Nat Cell Biol. 2018. PMID: 30050119 Free PMC article. Review.
-
Convergence of VEGF and YAP/TAZ signaling: Implications for angiogenesis and cancer biology.Sci Signal. 2018 Oct 16;11(552):eaau1165. doi: 10.1126/scisignal.aau1165. Sci Signal. 2018. PMID: 30327408 Free PMC article. Review.
-
The Laminin Receptors Basal Cell Adhesion Molecule/Lutheran and Integrin α7β1 on Human Hematopoietic Stem Cells.Front Cell Dev Biol. 2021 Oct 22;9:675240. doi: 10.3389/fcell.2021.675240. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34746117 Free PMC article.
References
-
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, et al. . 2005. A simplified laminin nomenclature. Matrix Biol 24: 326–332. - PubMed
-
- Beck B, Blanpain C. 2013. Unravelling cancer stem cell potential. Nat Rev Cancer 13: 727–738. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
