Bortezomib reduces pre-existing antibodies to recombinant immunotoxins in mice
- PMID: 25560410
- PMCID: PMC4323725
- DOI: 10.4049/jimmunol.1402324
Bortezomib reduces pre-existing antibodies to recombinant immunotoxins in mice
Abstract
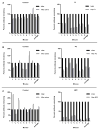
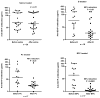
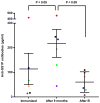
Recombinant immunotoxin (RIT) therapy is limited in patients by neutralizing Ab responses. Ninety percent of patients with normal immune systems make neutralizing Abs after one cycle of RIT, preventing repeated dosing. Furthermore, some patients have pre-existing Abs from environmental exposure to Pseudomonas exotoxin, the component of the RIT that elicits the neutralizing Ab response. Bortezomib is an U.S. Food and Drug Administration-approved proteasome inhibitor that selectively targets and kills plasma cells that are necessary for the neutralizing Ab response. We hypothesized that bortezomib may abrogate neutralizing Ab levels, making dosing of RIT possible in mice already immune to RIT. We immunized BALB/c mice with multiple doses of SS1P, a RIT whose Ab portion targets mesothelin. Mice with elevated Ab levels were separated into groups to receive saline, bortezomib, the pentostatin/cyclophosphamide (PC) regimen, or the bortezomib/PC (BPC) combination regimen. Four weeks after finishing therapy, plasma Ab levels were assayed, and bone marrow was harvested. The bortezomib and PC regimens significantly reduced Ab levels, and we observed fewer plasma cells in the bone marrow of bortezomib-treated mice but not in PC-treated mice. The BPC combination regimen almost completely eliminated Abs and further reduced plasma cells in the bone marrow. This regimen is more effective than individual regimens and may reduce Ab levels in patients with pre-existing neutralizing Abs to Pseudomonas exotoxin, allowing RIT treatment.
Figures

Similar articles
-
Immunogenicity of therapeutic recombinant immunotoxins.Immunol Rev. 2016 Mar;270(1):152-64. doi: 10.1111/imr.12390. Immunol Rev. 2016. PMID: 26864110 Free PMC article. Review.
-
Low-Dose Methotrexate Prevents Primary and Secondary Humoral Immune Responses and Induces Immune Tolerance to a Recombinant Immunotoxin.J Immunol. 2018 Mar 15;200(6):2038-2045. doi: 10.4049/jimmunol.1701430. Epub 2018 Feb 5. J Immunol. 2018. PMID: 29431691 Free PMC article.
-
Pentostatin plus cyclophosphamide safely and effectively prevents immunotoxin immunogenicity in murine hosts.Clin Cancer Res. 2011 Jun 1;17(11):3697-705. doi: 10.1158/1078-0432.CCR-11-0493. Epub 2011 Apr 26. Clin Cancer Res. 2011. PMID: 21521777 Free PMC article.
-
Characterization of the B cell epitopes associated with a truncated form of Pseudomonas exotoxin (PE38) used to make immunotoxins for the treatment of cancer patients.J Immunol. 2006 Dec 15;177(12):8822-34. doi: 10.4049/jimmunol.177.12.8822. J Immunol. 2006. PMID: 17142785
-
Immunogenicity of Immunotoxins Containing Pseudomonas Exotoxin A: Causes, Consequences, and Mitigation.Front Immunol. 2020 Jun 26;11:1261. doi: 10.3389/fimmu.2020.01261. eCollection 2020. Front Immunol. 2020. PMID: 32695104 Free PMC article. Review.
Cited by
-
A deimmunised form of the ribotoxin, α-sarcin, lacking CD4+ T cell epitopes and its use as an immunotoxin warhead.Protein Eng Des Sel. 2016 Nov 1;29(11):531-540. doi: 10.1093/protein/gzw045. Protein Eng Des Sel. 2016. PMID: 27578884 Free PMC article.
-
Mesothelin-Targeted Recombinant Immunotoxins for Solid Tumors.Biomolecules. 2020 Jun 28;10(7):973. doi: 10.3390/biom10070973. Biomolecules. 2020. PMID: 32605175 Free PMC article. Review.
-
Immunogenicity of therapeutic recombinant immunotoxins.Immunol Rev. 2016 Mar;270(1):152-64. doi: 10.1111/imr.12390. Immunol Rev. 2016. PMID: 26864110 Free PMC article. Review.
-
Comprehensive Single-Cell Immune Profiling Defines the Patient Multiple Myeloma Microenvironment Following Oncolytic Virus Therapy in a Phase Ib Trial.Clin Cancer Res. 2023 Dec 15;29(24):5087-5103. doi: 10.1158/1078-0432.CCR-23-0229. Clin Cancer Res. 2023. PMID: 37812476 Free PMC article. Clinical Trial.
-
Mucosal plasma cells are required to protect the upper airway and brain from infection.Immunity. 2022 Nov 8;55(11):2118-2134.e6. doi: 10.1016/j.immuni.2022.08.017. Epub 2022 Sep 21. Immunity. 2022. PMID: 36137543 Free PMC article.
References
-
- Schellekens H. Factors influencing the immunogenicity of therapeutic proteins. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2005;20(Suppl 6):vi3–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

