Survival and biodistribution of xenogenic adipose mesenchymal stem cells is not affected by the degree of inflammation in arthritis
- PMID: 25559623
- PMCID: PMC4283953
- DOI: 10.1371/journal.pone.0114962
Survival and biodistribution of xenogenic adipose mesenchymal stem cells is not affected by the degree of inflammation in arthritis
Erratum in
-
Correction: survival and biodistribution of xenogenic adipose mesenchymal stem cells is not affected by the degree of inflammation in arthritis.PLoS One. 2015 Mar 12;10(3):e0120406. doi: 10.1371/journal.pone.0120406. eCollection 2015. PLoS One. 2015. PMID: 25764005 Free PMC article. No abstract available.
Abstract
Background: Application of mesenchymal stem/stromal cells (MSCs) in treating different disorders, in particular osteo-articular diseases, is currently under investigation. We have already documented the safety of administrating human adipose tissue-derived stromal MSCs (hASCs) in immunodeficient mice. In the present study, we investigated whether the persistence of MSC is affected by the degree of inflammation and related to the therapeutic effect in two inflammatory models of arthritis.
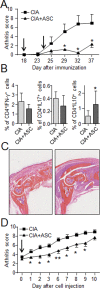
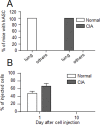
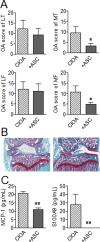
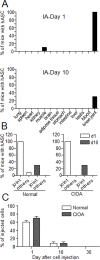
Methodology/principal findings: We used C57BL/6 or DBA/1 mice to develop collagenase-induced osteoarthritis (CIOA) or collagen-induced arthritis (CIA), respectively. Normal and diseased mice were administered 2.5×10(5) hASCs in the knee joints (i.a.) or 10(6) in the tail vein (i.v.). For CIA, clinical scores were monitored during the time course of the disease while for CIOA, OA scores were assessed by histology at euthanasia. Thirteen tissues were recovered at different time points and processed for real-time PCR and Alu sequence detection. Immunological analyses were performed at euthanasia. After i.v. infusion, no significant difference in the percentage of hASCs was quantified in the lungs of normal and CIA mice at day 1 while no cell was detected at day 10 taking into account the sensitivity of the assay, indicating that a high level of inflammation did not affect the persistence of cells. In CIOA mice, we reported the therapeutic efficacy of hASCs at reducing OA clinical scores at day 42 when hASCs were not detected in the joints. However, the percentage and distribution of hASCs were similar in osteoarthritic and normal mice at day 1 and 10 after implantation indicating that moderate inflammation does not alter hASC persistence in vivo.
Conclusions/significance: While inflammatory signals are required for the immunosuppressive function of MSCs, they do not enhance their capacity to survive in vivo, as evaluated in two xenogeneic inflammatory pre-clinical models of arthritis.
Conflict of interest statement
Figures
Similar articles
-
Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation.Arthritis Rheum. 2013 May;65(5):1181-93. doi: 10.1002/art.37894. Arthritis Rheum. 2013. PMID: 23400582 Free PMC article.
-
Administering human adipose-derived mesenchymal stem cells to prevent and treat experimental arthritis.Clin Immunol. 2011 Dec;141(3):328-37. doi: 10.1016/j.clim.2011.08.014. Epub 2011 Sep 2. Clin Immunol. 2011. PMID: 21944669
-
Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells.Arthritis Rheum. 2009 Apr;60(4):1006-19. doi: 10.1002/art.24405. Arthritis Rheum. 2009. PMID: 19333946
-
Mesenchymal stromal cells in rheumatoid arthritis: biological properties and clinical applications.Curr Stem Cell Res Ther. 2009 Jan;4(1):61-9. doi: 10.2174/157488809787169084. Curr Stem Cell Res Ther. 2009. PMID: 19149631 Review.
-
Mesenchymal stem cells and osteoarthritis: remedy or accomplice?Hum Gene Ther. 2010 Oct;21(10):1239-50. doi: 10.1089/hum.2010.138. Hum Gene Ther. 2010. PMID: 20649459 Review.
Cited by
-
Human embryonic stem cell-derived mesenchymal stromal cells ameliorate collagen-induced arthritis by inducing host-derived indoleamine 2,3 dioxygenase.Arthritis Res Ther. 2016 Apr 1;18:77. doi: 10.1186/s13075-016-0979-0. Arthritis Res Ther. 2016. PMID: 27036118 Free PMC article.
-
Cell-based therapies have disease-modifying effects on osteoarthritis in animal models. A systematic review by the ESSKA Orthobiologic Initiative. Part 1: adipose tissue-derived cell-based injectable therapies.Knee Surg Sports Traumatol Arthrosc. 2023 Feb;31(2):641-655. doi: 10.1007/s00167-022-07063-7. Epub 2022 Sep 14. Knee Surg Sports Traumatol Arthrosc. 2023. PMID: 36104484 Free PMC article. Review.
-
A Phase I Dose-Escalation Clinical Trial to Assess the Safety and Efficacy of Umbilical Cord-Derived Mesenchymal Stromal Cells in Knee Osteoarthritis.Stem Cells Transl Med. 2024 Mar 15;13(3):193-203. doi: 10.1093/stcltm/szad088. Stem Cells Transl Med. 2024. PMID: 38366909 Free PMC article. Clinical Trial.
-
Micromolding-based encapsulation of mesenchymal stromal cells in alginate for intraarticular injection in osteoarthritis.Mater Today Bio. 2023 Feb 13;19:100581. doi: 10.1016/j.mtbio.2023.100581. eCollection 2023 Apr. Mater Today Bio. 2023. PMID: 36896417 Free PMC article.
-
Intra-articularly injected mesenchymal stem cells promote cartilage regeneration, but do not permanently engraft in distant organs.Sci Rep. 2019 Jul 12;9(1):10153. doi: 10.1038/s41598-019-46554-5. Sci Rep. 2019. PMID: 31300685 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

