Differential drug resistance acquisition to doxorubicin and paclitaxel in breast cancer cells
- PMID: 25550688
- PMCID: PMC4279688
- DOI: 10.1186/s12935-014-0142-4
Differential drug resistance acquisition to doxorubicin and paclitaxel in breast cancer cells
Abstract
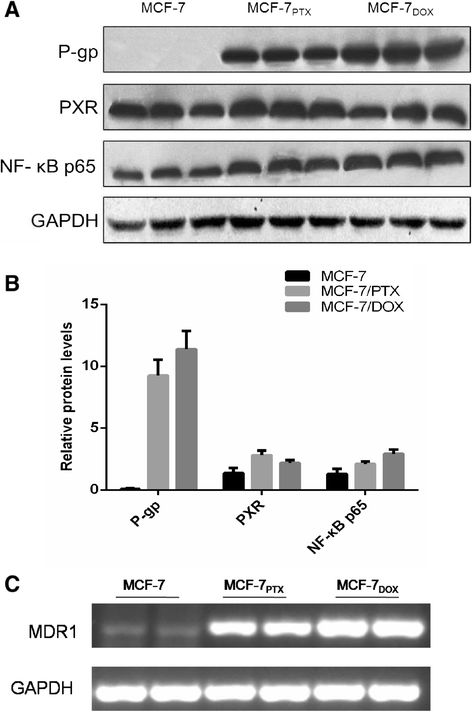
Background: Several signal transduction pathways have been reported being involved in the acquisition of P-glycoprotein (P-gp) mediated multi-drug resistance (MDR) upon exposure to anti-cancer drugs, whereas there is evidence indicating that the expression and activity of P-gp were not equally or even reversely modulated by different drugs.
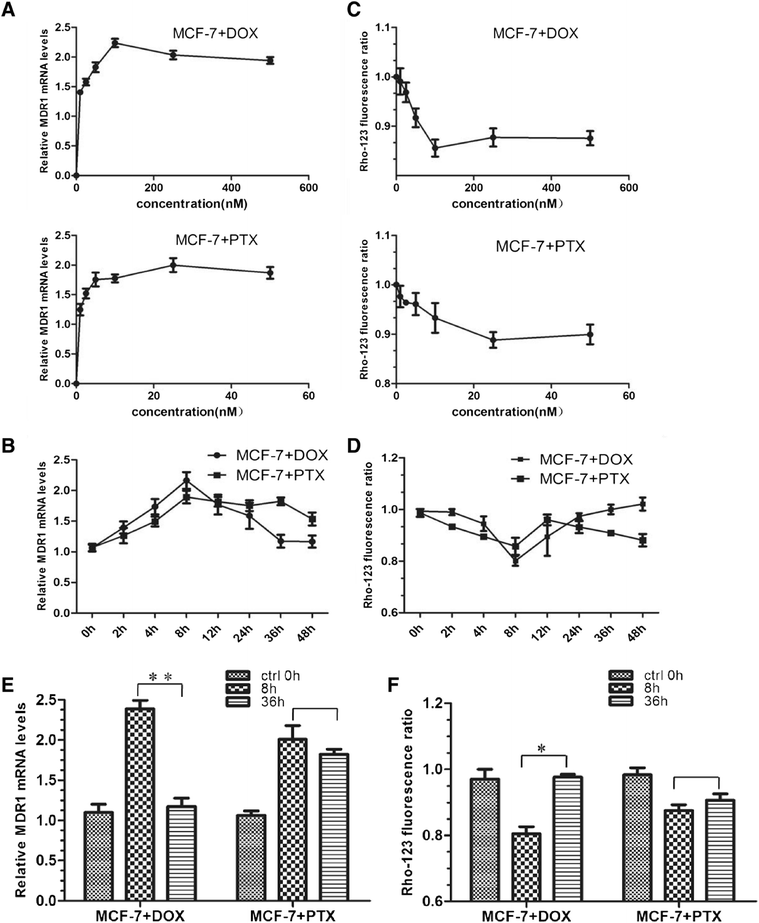
Methods: To further illustrate this drug-specific effect, possible mechanisms that enable breast cancer cells MCF-7 to acquire MDR to either paclitaxel (PTX) or doxorubicin (DOX) were investigated in a time-dependent manner.
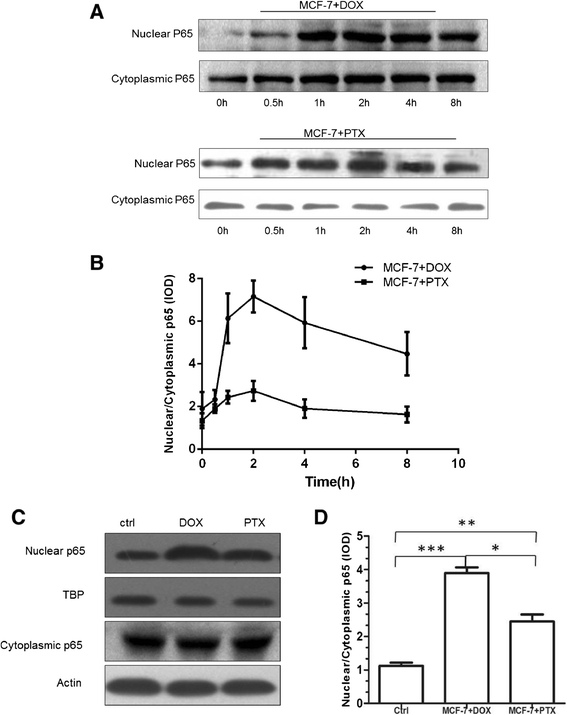
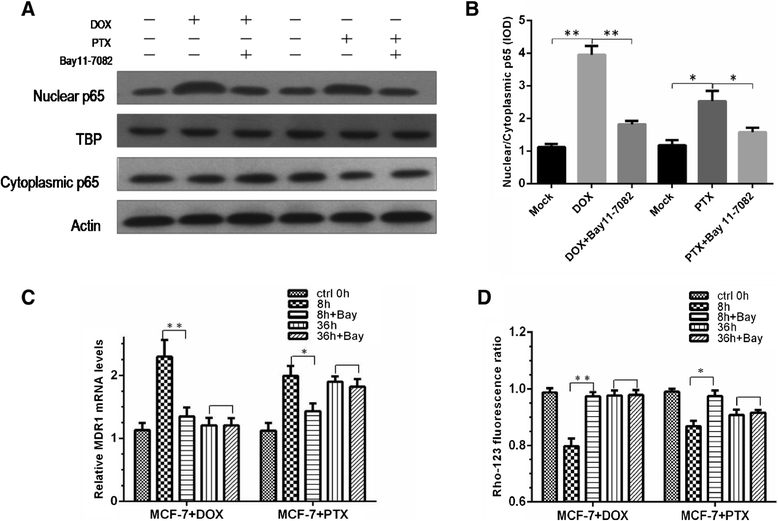
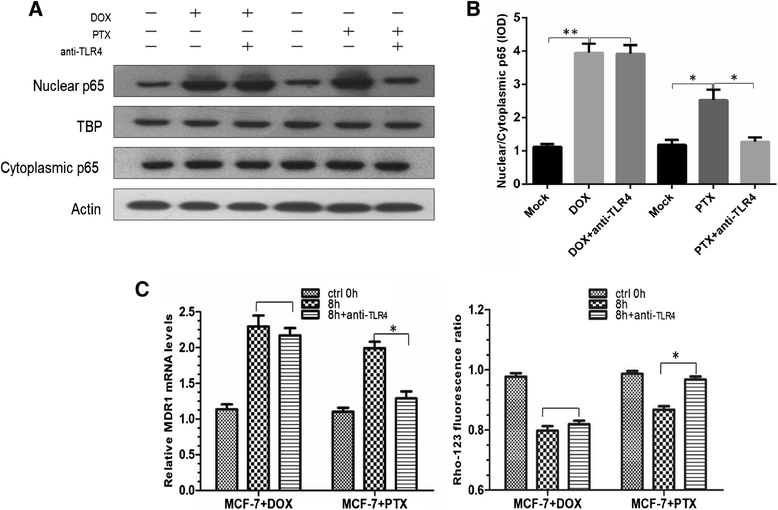
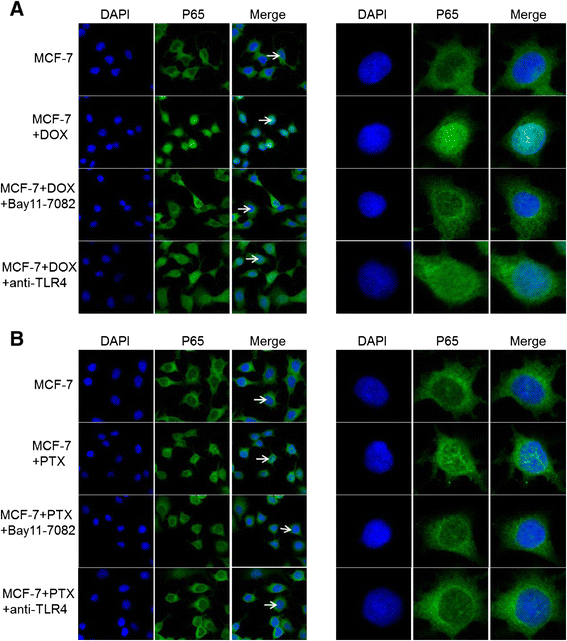
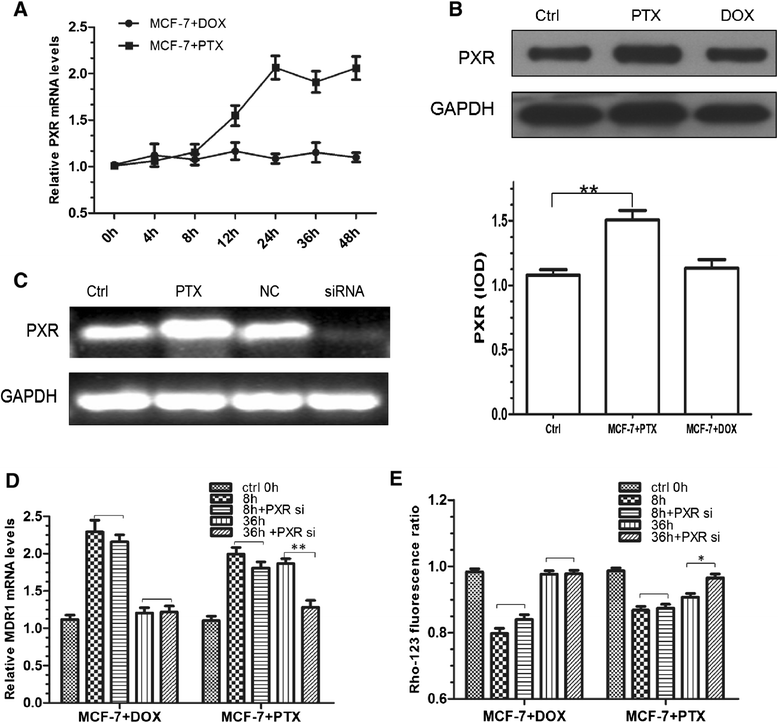
Results: The results suggested that at least two pathways participated in this process. One was the short and transient activation of NF-κB, the second one was the relatively prolonged induction of PXR. Both PXR and NF-κB pathways took part in the PTX drug resistance acquisition, whereas DOX did not exert a significant effect on the PXR-mediated induction of P-gp. Furthermore, the property of NF-κB activation shared by DOX and PTX was not identical. An attempt made in the present study demonstrated that the acquired resistance to DOX was via or partially via NF-κB activation but not its upstream receptor TLR4, while PTX can induce the drug resistance via TLR4-NF-κB pathway.
Conclusions: To our knowledge, this report is among the first to directly compare the time dependence of NF-κB and PXR pathways. The current study provides useful insight into the distinct ability of DOX and PTX to induce P-gp mediated MDR in breast cancer. Different strategies may be required to circumvent MDR in the presence of different anti-cancer drugs.
Keywords: Doxorubicin (DOX); Drug-specific; Multi-drug Resistance Acquisition (MDR); P-glycoprotein (P-gp); Paclitaxel (PTX).
Figures
Similar articles
-
Parthenolide reverses doxorubicin resistance in human lung carcinoma A549 cells by attenuating NF-κB activation and HSP70 up-regulation.Toxicol Lett. 2013 Aug 14;221(2):73-82. doi: 10.1016/j.toxlet.2013.06.215. Epub 2013 Jun 20. Toxicol Lett. 2013. PMID: 23792430
-
Human pharmacokinetic characterization and in vitro study of the interaction between doxorubicin and paclitaxel in patients with breast cancer.J Clin Oncol. 1997 May;15(5):1906-15. doi: 10.1200/JCO.1997.15.5.1906. J Clin Oncol. 1997. PMID: 9164201
-
Schisandrin A reverses doxorubicin-resistant human breast cancer cell line by the inhibition of P65 and Stat3 phosphorylation.Breast Cancer. 2018 Mar;25(2):233-242. doi: 10.1007/s12282-017-0821-9. Epub 2017 Nov 27. Breast Cancer. 2018. PMID: 29181822
-
Chloride channel-3 mediates multidrug resistance of cancer by upregulating P-glycoprotein expression.J Cell Physiol. 2019 May;234(5):6611-6623. doi: 10.1002/jcp.27402. Epub 2018 Sep 19. J Cell Physiol. 2019. PMID: 30230544
-
Pluronic-based functional polymeric mixed micelles for co-delivery of doxorubicin and paclitaxel to multidrug resistant tumor.Int J Pharm. 2015 Jul 5;488(1-2):44-58. doi: 10.1016/j.ijpharm.2015.04.048. Epub 2015 Apr 18. Int J Pharm. 2015. PMID: 25899286
Cited by
-
Glucose-dependent regulation of pregnane X receptor is modulated by AMP-activated protein kinase.Sci Rep. 2017 Apr 24;7:46751. doi: 10.1038/srep46751. Sci Rep. 2017. PMID: 28436464 Free PMC article.
-
Overexpression of microRNA-24 increases the sensitivity to paclitaxel in drug-resistant breast carcinoma cell lines via targeting ABCB9.Oncol Lett. 2016 Nov;12(5):3905-3911. doi: 10.3892/ol.2016.5139. Epub 2016 Sep 15. Oncol Lett. 2016. PMID: 27895747 Free PMC article.
-
MiR-122 Reverses the Doxorubicin-Resistance in Hepatocellular Carcinoma Cells through Regulating the Tumor Metabolism.PLoS One. 2016 May 3;11(5):e0152090. doi: 10.1371/journal.pone.0152090. eCollection 2016. PLoS One. 2016. PMID: 27138141 Free PMC article.
-
Pyrophen Isolated from the Endophytic Fungus Aspergillus fumigatus Strain KARSV04 Synergizes the Effect of Doxorubicin in Killing MCF7 but not T47D Cells.Turk J Pharm Sci. 2020 Jun;17(3):280-284. doi: 10.4274/tjps.galenos.2019.30633. Epub 2020 Jun 22. Turk J Pharm Sci. 2020. PMID: 32636705 Free PMC article.
-
MiRNA-449 family is epigenetically repressed and sensitizes to doxorubicin through ACSL4 downregulation in triple-negative breast cancer.Cell Death Discov. 2024 Aug 22;10(1):372. doi: 10.1038/s41420-024-02128-7. Cell Death Discov. 2024. PMID: 39174500 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

