Subunit determination of the conductance of hair-cell mechanotransducer channels
- PMID: 25550511
- PMCID: PMC4321290
- DOI: 10.1073/pnas.1420906112
Subunit determination of the conductance of hair-cell mechanotransducer channels
Abstract
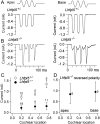
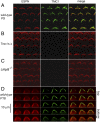
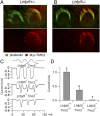
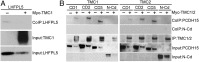
Cochlear hair cells convert sound stimuli into electrical signals by gating of mechanically sensitive ion channels in their stereociliary (hair) bundle. The molecular identity of this ion channel is still unclear, but its properties are modulated by accessory proteins. Two such proteins are transmembrane channel-like protein isoform 1 (TMC1) and tetraspan membrane protein of hair cell stereocilia (TMHS, also known as lipoma HMGIC fusion partner-like 5, LHFPL5), both thought to be integral components of the mechanotransduction machinery. Here we show that, in mice harboring an Lhfpl5 null mutation, the unitary conductance of outer hair cell mechanotransducer (MT) channels was reduced relative to wild type, and the tonotopic gradient in conductance, where channels from the cochlear base are nearly twice as conducting as those at the apex, was almost absent. The macroscopic MT current in these mutants was attenuated and the tonotopic gradient in amplitude was also lost, although the current was not completely extinguished. The consequences of Lhfpl5 mutation mirror those due to Tmc1 mutation, suggesting a part of the MT-channel conferring a large and tonotopically variable conductance is similarly disrupted in the absence of Lhfpl5 or Tmc1. Immunolabelling demonstrated TMC1 throughout the stereociliary bundles in wild type but not in Lhfpl5 mutants, implying the channel effect of Lhfpl5 mutations stems from down-regulation of TMC1. Both LHFPL5 and TMC1 were shown to interact with protocadherin-15, a component of the tip link, which applies force to the MT channel. We propose that titration of the TMC1 content of the MT channel sets the gradient in unitary conductance along the cochlea.
Keywords: LHFPL5; TMC1; cochlea; hair cell; mechanotransducer channels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
How many proteins does it take to gate hair cell mechanotransduction?Proc Natl Acad Sci U S A. 2015 Feb 3;112(5):1254-5. doi: 10.1073/pnas.1424987112. Epub 2015 Jan 23. Proc Natl Acad Sci U S A. 2015. PMID: 25617369 Free PMC article. No abstract available.
Similar articles
-
Conductance and block of hair-cell mechanotransducer channels in transmembrane channel-like protein mutants.J Gen Physiol. 2014 Jul;144(1):55-69. doi: 10.1085/jgp.201411173. J Gen Physiol. 2014. PMID: 24981230 Free PMC article.
-
LHFPL5 is a key element in force transmission from the tip link to the hair cell mechanotransducer channel.Proc Natl Acad Sci U S A. 2024 Jan 16;121(3):e2318270121. doi: 10.1073/pnas.2318270121. Epub 2024 Jan 9. Proc Natl Acad Sci U S A. 2024. PMID: 38194445 Free PMC article.
-
Localization of TMC1 and LHFPL5 in auditory hair cells in neonatal and adult mice.FASEB J. 2019 Jun;33(6):6838-6851. doi: 10.1096/fj.201802155RR. Epub 2019 Feb 26. FASEB J. 2019. PMID: 30808210
-
Is TMC1 the Hair Cell Mechanotransducer Channel?Biophys J. 2016 Jul 12;111(1):3-9. doi: 10.1016/j.bpj.2016.05.032. Biophys J. 2016. PMID: 27410728 Free PMC article. Review.
-
Mechanically Gated Ion Channels in Mammalian Hair Cells.Front Cell Neurosci. 2018 Apr 11;12:100. doi: 10.3389/fncel.2018.00100. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29755320 Free PMC article. Review.
Cited by
-
New Tmc1 Deafness Mutations Impact Mechanotransduction in Auditory Hair Cells.J Neurosci. 2021 May 19;41(20):4378-4391. doi: 10.1523/JNEUROSCI.2537-20.2021. Epub 2021 Apr 6. J Neurosci. 2021. PMID: 33824189 Free PMC article.
-
Alternative Splicing of Three Genes Encoding Mechanotransduction-Complex Proteins in Auditory Hair Cells.eNeuro. 2021 Feb 23;8(1):ENEURO.0381-20.2020. doi: 10.1523/ENEURO.0381-20.2020. Print 2021 Jan-Feb. eNeuro. 2021. PMID: 33509951 Free PMC article.
-
Sensory Hair Cells: An Introduction to Structure and Physiology.Integr Comp Biol. 2018 Aug 1;58(2):282-300. doi: 10.1093/icb/icy064. Integr Comp Biol. 2018. PMID: 29917041 Free PMC article. Review.
-
How Transmembrane Inner Ear (TMIE) plays role in the auditory system: A mystery to us.J Cell Mol Med. 2021 May 13;25(13):5869-83. doi: 10.1111/jcmm.16610. Online ahead of print. J Cell Mol Med. 2021. PMID: 33987950 Free PMC article. Review.
-
cAMP and voltage modulate rat auditory mechanotransduction by decreasing the stiffness of gating springs.Proc Natl Acad Sci U S A. 2022 Jul 26;119(30):e2107567119. doi: 10.1073/pnas.2107567119. Epub 2022 Jul 19. Proc Natl Acad Sci U S A. 2022. PMID: 35858439 Free PMC article.
References
-
- Pickles JO, Comis SD, Osborne MP. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear Res. 1984;15(2):103–112. - PubMed
-
- Furness DN, Katori Y, Nirmal Kumar B, Hackney CM. The dimensions and structural attachments of tip links in mammalian cochlear hair cells and the effects of exposure to different levels of extracellular calcium. Neuroscience. 2008;154(1):10–21. - PubMed
-
- Kazmierczak P, et al. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature. 2007;449(7158):87–91. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

