Lysosomal sequestration of hydrophobic weak base chemotherapeutics triggers lysosomal biogenesis and lysosome-dependent cancer multidrug resistance
- PMID: 25544758
- PMCID: PMC4359223
- DOI: 10.18632/oncotarget.2732
Lysosomal sequestration of hydrophobic weak base chemotherapeutics triggers lysosomal biogenesis and lysosome-dependent cancer multidrug resistance
Erratum in
-
Correction: Lysosomal sequestration of hydrophobic weak base chemotherapeutics triggers lysosomal biogenesis and lysosome-dependent cancer multidrug resistance.Oncotarget. 2022 Apr 1;13:585-586. doi: 10.18632/oncotarget.28110. eCollection 2022. Oncotarget. 2022. PMID: 35391719 Free PMC article.
Abstract
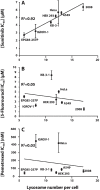
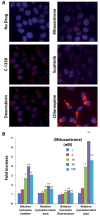
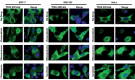
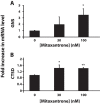
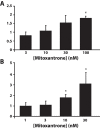
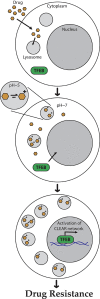
Multidrug resistance (MDR) is a primary hindrance to curative cancer chemotherapy. In this respect, lysosomes were suggested to play a role in intrinsic MDR by sequestering protonated hydrophobic weak base chemotherapeutics away from their intracellular target sites. Here we show that intrinsic resistance to sunitinib, a hydrophobic weak base tyrosine kinase inhibitor known to accumulate in lysosomes, tightly correlates with the number of lysosomes accumulating high levels of sunitinib in multiple human carcinoma cells. Furthermore, exposure of cancer cells to hydrophobic weak base drugs leads to a marked increase in the number of lysosomes per cell. Non-cytotoxic, nanomolar concentrations, of the hydrophobic weak base chemotherapeutics doxorubicin and mitoxantrone triggered rapid lysosomal biogenesis that was associated with nuclear translocation of TFEB, the dominant transcription factor regulating lysosomal biogenesis. This resulted in increased lysosomal gene expression and lysosomal enzyme activity. Thus, treatment of cancer cells with hydrophobic weak base chemotherapeutics and their consequent sequestration in lysosomes triggers lysosomal biogenesis, thereby further enhancing lysosomal drug entrapment and MDR. The current study provides the first evidence that drug-induced TFEB-associated lysosomal biogenesis is an emerging determinant of MDR and suggests that circumvention of lysosomal drug sequestration is a novel strategy to overcome this chemoresistance.
Figures
Similar articles
-
Lysosomes as mediators of drug resistance in cancer.Drug Resist Updat. 2016 Jan;24:23-33. doi: 10.1016/j.drup.2015.11.004. Epub 2015 Nov 26. Drug Resist Updat. 2016. PMID: 26830313 Review.
-
The role of endolysosomal trafficking in anticancer drug resistance.Drug Resist Updat. 2021 Jul;57:100769. doi: 10.1016/j.drup.2021.100769. Epub 2021 Jun 2. Drug Resist Updat. 2021. PMID: 34217999 Review.
-
Lysosomal sequestration of weak base drugs, lysosomal biogenesis, and cell cycle alteration.Biomed Pharmacother. 2022 Sep;153:113328. doi: 10.1016/j.biopha.2022.113328. Epub 2022 Jul 1. Biomed Pharmacother. 2022. PMID: 35785701
-
Lysosomal accumulation of anticancer drugs triggers lysosomal exocytosis.Oncotarget. 2017 Jul 11;8(28):45117-45132. doi: 10.18632/oncotarget.15155. Oncotarget. 2017. PMID: 28187461 Free PMC article.
-
Lysosomal Fusion: An Efficient Mechanism Increasing Their Sequestration Capacity for Weak Base Drugs without Apparent Lysosomal Biogenesis.Biomolecules. 2020 Jan 3;10(1):77. doi: 10.3390/biom10010077. Biomolecules. 2020. PMID: 31947839 Free PMC article.
Cited by
-
FDA approved drugs with pharmacotherapeutic potential for SARS-CoV-2 (COVID-19) therapy.Drug Resist Updat. 2020 Dec;53:100719. doi: 10.1016/j.drup.2020.100719. Epub 2020 Jul 15. Drug Resist Updat. 2020. PMID: 32717568 Free PMC article. Review.
-
Cytosolic Delivery of Macromolecules in Live Human Cells Using the Combined Endosomal Escape Activities of a Small Molecule and Cell Penetrating Peptides.ACS Chem Biol. 2019 Dec 20;14(12):2641-2651. doi: 10.1021/acschembio.9b00585. Epub 2019 Oct 31. ACS Chem Biol. 2019. PMID: 31633910 Free PMC article.
-
Anticancer drug resistance: An update and perspective.Drug Resist Updat. 2021 Dec;59:100796. doi: 10.1016/j.drup.2021.100796. Epub 2021 Dec 16. Drug Resist Updat. 2021. PMID: 34953682 Free PMC article. Review.
-
Hydroxychloroquine: A Physiologically-Based Pharmacokinetic Model in the Context of Cancer-Related Autophagy Modulation.J Pharmacol Exp Ther. 2018 Jun;365(3):447-459. doi: 10.1124/jpet.117.245639. Epub 2018 Feb 8. J Pharmacol Exp Ther. 2018. PMID: 29438998 Free PMC article.
-
Update on Autophagy Inhibitors in Cancer: Opening up to a Therapeutic Combination with Immune Checkpoint Inhibitors.Cells. 2023 Jun 23;12(13):1702. doi: 10.3390/cells12131702. Cells. 2023. PMID: 37443736 Free PMC article. Review.
References
-
- Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. - PubMed
-
- Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. - PubMed
-
- Gonen N, Assaraf YG. Antifolates in cancer therapy: structure, activity and mechanisms of drug resistance. Drug Resist Updat. 2012;15:183–210. - PubMed
-
- Assaraf YG. The role of multidrug resistance efflux transporters in antifolate resistance and folate homeostasis. Drug Resist Updat. 2006;9:227–246. - PubMed
-
- Assaraf YG. Molecular basis of antifolate resistance. Cancer Metastasis Rev. 2007;26:153–181. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

