mTORC1 phosphorylates UVRAG to negatively regulate autophagosome and endosome maturation
- PMID: 25533187
- PMCID: PMC4304967
- DOI: 10.1016/j.molcel.2014.11.013
mTORC1 phosphorylates UVRAG to negatively regulate autophagosome and endosome maturation
Abstract
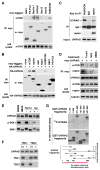
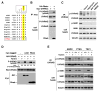
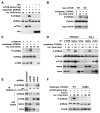
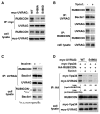
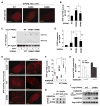
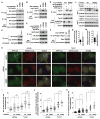
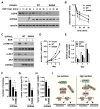
mTORC1 plays a key role in autophagy as a negative regulator. The currently known targets of mTORC1 in the autophagy pathway mainly function at early stages of autophagosome formation. Here, we identify that mTORC1 inhibits later stages of autophagy by phosphorylating UVRAG. Under nutrient-enriched conditions, mTORC1 binds and phosphorylates UVRAG. The phosphorylation positively regulates the association of UVRAG with RUBICON, thereby enhancing the antagonizing effect of RUBICON on UVRAG-mediated autophagosome maturation. Upon dephosphorylation, UVRAG is released from RUBICON to interact with the HOPS complex, a component for the late endosome and lysosome fusion machinery, and enhances autophagosome and endosome maturation. Consequently, the dephosphorylation of UVRAG facilitates the lysosomal degradation of epidermal growth factor receptor (EGFR), reduces EGFR signaling, and suppresses cancer cell proliferation and tumor growth. These results demonstrate that mTORC1 engages in late stages of autophagy and endosome maturation, defining a broader range of mTORC1 functions in the membrane-associated processes.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
Comment in
-
ZMP: a master regulator of one-carbon metabolism.Mol Cell. 2015 Jan 22;57(2):203-4. doi: 10.1016/j.molcel.2015.01.012. Mol Cell. 2015. PMID: 25616065 Free PMC article.
Similar articles
-
Ubiquitination of UVRAG by SMURF1 promotes autophagosome maturation and inhibits hepatocellular carcinoma growth.Autophagy. 2019 Jul;15(7):1130-1149. doi: 10.1080/15548627.2019.1570063. Epub 2019 Jan 27. Autophagy. 2019. PMID: 30686098 Free PMC article.
-
Pacer Mediates the Function of Class III PI3K and HOPS Complexes in Autophagosome Maturation by Engaging Stx17.Mol Cell. 2017 Mar 16;65(6):1029-1043.e5. doi: 10.1016/j.molcel.2017.02.010. Mol Cell. 2017. PMID: 28306502
-
Beclin1-binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking.Nat Cell Biol. 2008 Jul;10(7):776-87. doi: 10.1038/ncb1740. Epub 2008 Jun 15. Nat Cell Biol. 2008. PMID: 18552835 Free PMC article.
-
Key mediators of intracellular amino acids signaling to mTORC1 activation.Amino Acids. 2015 May;47(5):857-67. doi: 10.1007/s00726-015-1937-x. Epub 2015 Feb 21. Amino Acids. 2015. PMID: 25701492 Review.
-
The autophagosome: origins unknown, biogenesis complex.Nat Rev Mol Cell Biol. 2013 Dec;14(12):759-74. doi: 10.1038/nrm3696. Epub 2013 Nov 8. Nat Rev Mol Cell Biol. 2013. PMID: 24201109 Review.
Cited by
-
The roles of the inhibitory autophagy regulator Rubicon in the heart: A new therapeutic target to prevent cardiac cell death.Exp Mol Med. 2021 Apr;53(4):528-536. doi: 10.1038/s12276-021-00600-3. Epub 2021 Apr 14. Exp Mol Med. 2021. PMID: 33854187 Free PMC article. Review.
-
p27 controls Ragulator and mTOR activity in amino acid-deprived cells to regulate the autophagy-lysosomal pathway and coordinate cell cycle and cell growth.Nat Cell Biol. 2020 Sep;22(9):1076-1090. doi: 10.1038/s41556-020-0554-4. Epub 2020 Aug 17. Nat Cell Biol. 2020. PMID: 32807902
-
Molecular Mechanism and Regulation of Autophagy and Its Potential Role in Epilepsy.Cells. 2022 Aug 23;11(17):2621. doi: 10.3390/cells11172621. Cells. 2022. PMID: 36078029 Free PMC article. Review.
-
The early diagnosis and pathogenic mechanisms of sepsis-related acute kidney injury.Open Life Sci. 2023 Aug 31;18(1):20220700. doi: 10.1515/biol-2022-0700. eCollection 2023. Open Life Sci. 2023. PMID: 37671089 Free PMC article. Review.
-
Autophagy: A Friend or Foe in Allergic Asthma?Int J Mol Sci. 2021 Jun 12;22(12):6314. doi: 10.3390/ijms22126314. Int J Mol Sci. 2021. PMID: 34204710 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

