Role and species-specific expression of colon T cell homing receptor GPR15 in colitis
- PMID: 25531831
- PMCID: PMC4338558
- DOI: 10.1038/ni.3079
Role and species-specific expression of colon T cell homing receptor GPR15 in colitis
Abstract
Lymphocyte recruitment maintains intestinal immune homeostasis but also contributes to inflammation. The orphan chemoattractant receptor GPR15 mediates regulatory T cell homing and immunosuppression in the mouse colon. We show that GPR15 is also expressed by mouse TH17 and TH1 effector cells and is required for colitis in a model that depends on the trafficking of these cells to the colon. In humans GPR15 is expressed by effector cells, including pathogenic TH2 cells in ulcerative colitis, but is expressed poorly or not at all by colon regulatory T (Treg) cells. The TH2 transcriptional activator GATA-3 and the Treg-associated transcriptional repressor FOXP3 robustly bind human, but not mouse, GPR15 enhancer sequences, correlating with receptor expression. Our results highlight species differences in GPR15 regulation and suggest it as a potential therapeutic target for colitis.
Figures
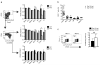

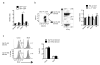
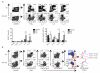


Comment in
-
GPR15: a tale of two species.Nat Immunol. 2015 Feb;16(2):137-9. doi: 10.1038/ni.3084. Nat Immunol. 2015. PMID: 25594457 No abstract available.
Similar articles
-
GPR15: a tale of two species.Nat Immunol. 2015 Feb;16(2):137-9. doi: 10.1038/ni.3084. Nat Immunol. 2015. PMID: 25594457 No abstract available.
-
GPR15-mediated homing controls immune homeostasis in the large intestine mucosa.Science. 2013 Jun 21;340(6139):1456-9. doi: 10.1126/science.1237013. Epub 2013 May 9. Science. 2013. PMID: 23661644 Free PMC article.
-
The aryl hydrocarbon receptor regulates expression of mucosal trafficking receptor GPR15.Mucosal Immunol. 2021 Jul;14(4):852-861. doi: 10.1038/s41385-021-00390-x. Epub 2021 Mar 5. Mucosal Immunol. 2021. PMID: 33674764 Free PMC article.
-
Emerging roles of a chemoattractant receptor GPR15 and ligands in pathophysiology.Front Immunol. 2023 Jun 30;14:1179456. doi: 10.3389/fimmu.2023.1179456. eCollection 2023. Front Immunol. 2023. PMID: 37457732 Free PMC article. Review.
-
Leukocyte Trafficking to the Small Intestine and Colon.Gastroenterology. 2016 Feb;150(2):340-54. doi: 10.1053/j.gastro.2015.10.046. Epub 2015 Nov 6. Gastroenterology. 2016. PMID: 26551552 Free PMC article. Review.
Cited by
-
GPR15 in colon cancer development and anti-tumor immune responses.Front Oncol. 2023 Nov 24;13:1254307. doi: 10.3389/fonc.2023.1254307. eCollection 2023. Front Oncol. 2023. PMID: 38074634 Free PMC article.
-
GPR15: a tale of two species.Nat Immunol. 2015 Feb;16(2):137-9. doi: 10.1038/ni.3084. Nat Immunol. 2015. PMID: 25594457 No abstract available.
-
Different Disease Endotypes in Phenotypically Similar Vasculitides Affecting Small-to-Medium Sized Blood Vessels.Front Immunol. 2021 Feb 22;12:638571. doi: 10.3389/fimmu.2021.638571. eCollection 2021. Front Immunol. 2021. PMID: 33692808 Free PMC article.
-
Aberrant hepatic trafficking of gut-derived T cells is not specific to primary sclerosing cholangitis.Hepatology. 2022 Mar;75(3):518-530. doi: 10.1002/hep.32193. Epub 2021 Dec 7. Hepatology. 2022. PMID: 34633679 Free PMC article.
-
CD4+ T-cell subsets in inflammatory diseases: beyond the Th1/Th2 paradigm.Int Immunol. 2016 Apr;28(4):163-71. doi: 10.1093/intimm/dxw006. Epub 2016 Feb 12. Int Immunol. 2016. PMID: 26874355 Free PMC article. Review.
References
-
- Mora JR, Von Andrian UH. Specificity and plasticity of memory lymphocyte migration. Curr Top Microbiol Immunol. 2006;308:83–116. - PubMed
-
- Zabel BA, Rott A, Butcher EC. Leukocyte Chemoattractant Receptors in Human Disease Pathogenesis. Annual review of pathology. 2014 doi:10.1146/annurev-pathol-012513-104640. - PubMed
-
- Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–28. doi:10.1152/ajpregu.00738.2001. - PubMed
-
- Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16:1–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI07290/AI/NIAID NIH HHS/United States
- R37 AI047822/AI/NIAID NIH HHS/United States
- K08 DK069385/DK/NIDDK NIH HHS/United States
- 1F32 AI082924/AI/NIAID NIH HHS/United States
- R01 AI109452/AI/NIAID NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R03 DK085426/DK/NIDDK NIH HHS/United States
- 091009/WT_/Wellcome Trust/United Kingdom
- G0802068/CRUK_/Cancer Research UK/United Kingdom
- T32 AI007290/AI/NIAID NIH HHS/United States
- F32 AI082924/AI/NIAID NIH HHS/United States
- T32 HL098049/HL/NHLBI NIH HHS/United States
- G0802068/MRC_/Medical Research Council/United Kingdom
- DK56339/DK/NIDDK NIH HHS/United States
- AI109452A/AI/NIAID NIH HHS/United States
- P30 DK056339/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

