Interleukin-17 receptor a signaling in transformed enterocytes promotes early colorectal tumorigenesis
- PMID: 25526314
- PMCID: PMC4272447
- DOI: 10.1016/j.immuni.2014.11.009
Interleukin-17 receptor a signaling in transformed enterocytes promotes early colorectal tumorigenesis
Abstract
Interleukin-17A (IL-17A) is a pro-inflammatory cytokine linked to rapid malignant progression of colorectal cancer (CRC) and therapy resistance. IL-17A exerts its pro-tumorigenic activity through its type A receptor (IL-17RA). However, IL-17RA is expressed in many cell types, including hematopoietic, fibroblastoid, and epithelial cells, in the tumor microenvironment, and how IL-17RA engagement promotes colonic tumorigenesis is unknown. Here we show that IL-17RA signals directly within transformed colonic epithelial cells (enterocytes) to promote early tumor development. IL-17RA engagement activates ERK, p38 MAPK, and NF-κB signaling and promotes the proliferation of tumorigenic enterocytes that just lost expression of the APC tumor suppressor. Although IL-17RA signaling also controls the production of IL-6, this mechanism makes only a partial contribution to colonic tumorigenesis. Combined treatment with chemotherapy, which induces IL-17A expression, and an IL-17A neutralizing antibody enhanced the therapeutic responsiveness of established colon tumors. These findings establish IL-17A and IL-17RA as therapeutic targets in colorectal cancer.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
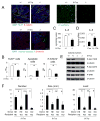
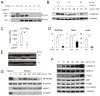
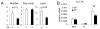
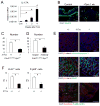
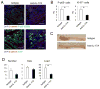
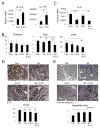
Comment in
-
IL-17 cuts to the chase in colon cancer.Immunity. 2014 Dec 18;41(6):880-2. doi: 10.1016/j.immuni.2014.12.004. Immunity. 2014. PMID: 25526302
Similar articles
-
IL-17A/IL-17RA promotes invasion and activates MMP-2 and MMP-9 expression via p38 MAPK signaling pathway in non-small cell lung cancer.Mol Cell Biochem. 2019 May;455(1-2):195-206. doi: 10.1007/s11010-018-3483-9. Epub 2018 Dec 18. Mol Cell Biochem. 2019. PMID: 30564960 Clinical Trial.
-
IL-17 cuts to the chase in colon cancer.Immunity. 2014 Dec 18;41(6):880-2. doi: 10.1016/j.immuni.2014.12.004. Immunity. 2014. PMID: 25526302
-
IL-17 signaling in steatotic hepatocytes and macrophages promotes hepatocellular carcinoma in alcohol-related liver disease.J Hepatol. 2020 May;72(5):946-959. doi: 10.1016/j.jhep.2019.12.016. Epub 2019 Dec 31. J Hepatol. 2020. PMID: 31899206 Free PMC article.
-
An Overview of Interleukin-17A and Interleukin-17 Receptor A Structure, Interaction and Signaling.Protein Pept Lett. 2015;22(7):570-8. doi: 10.2174/0929866522666150520145554. Protein Pept Lett. 2015. PMID: 25990083 Review.
-
Interleukin-17A in lipid metabolism and atherosclerosis.Clin Chim Acta. 2014 Apr 20;431:33-9. doi: 10.1016/j.cca.2014.01.012. Epub 2014 Feb 6. Clin Chim Acta. 2014. PMID: 24508995 Review.
Cited by
-
Src Family Tyrosine Kinases in Intestinal Homeostasis, Regeneration and Tumorigenesis.Cancers (Basel). 2020 Jul 23;12(8):2014. doi: 10.3390/cancers12082014. Cancers (Basel). 2020. PMID: 32717909 Free PMC article. Review.
-
IL-17A promotes fatty acid uptake through the IL-17A/IL-17RA/p-STAT3/FABP4 axis to fuel ovarian cancer growth in an adipocyte-rich microenvironment.Cancer Immunol Immunother. 2020 Jan;69(1):115-126. doi: 10.1007/s00262-019-02445-2. Epub 2019 Dec 4. Cancer Immunol Immunother. 2020. PMID: 31802182 Free PMC article.
-
Safety and danger of biologic treatments in psoriasis in context of cutaneous T-cell lymphoma (CTCL).Postepy Dermatol Alergol. 2021 Dec;38(6):953-960. doi: 10.5114/ada.2021.107553. Epub 2021 Oct 25. Postepy Dermatol Alergol. 2021. PMID: 35126000 Free PMC article. Review.
-
IL-17A/IL-17RA promotes invasion and activates MMP-2 and MMP-9 expression via p38 MAPK signaling pathway in non-small cell lung cancer.Mol Cell Biochem. 2019 May;455(1-2):195-206. doi: 10.1007/s11010-018-3483-9. Epub 2018 Dec 18. Mol Cell Biochem. 2019. PMID: 30564960 Clinical Trial.
-
Regulation of intestinal immunity by dietary fatty acids.Mucosal Immunol. 2022 May;15(5):846-856. doi: 10.1038/s41385-022-00547-2. Epub 2022 Jul 12. Mucosal Immunol. 2022. PMID: 35821290 Review.
References
-
- Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. The New England journal of medicine. 2004;350:2343–2351. - PubMed
-
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. - PubMed
-
- Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501. - PubMed
-
- Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer cell. 2009;15:91–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

