The role of the NADPH oxidase NOX2 in prion pathogenesis
- PMID: 25502554
- PMCID: PMC4263757
- DOI: 10.1371/journal.ppat.1004531
The role of the NADPH oxidase NOX2 in prion pathogenesis
Abstract
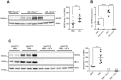
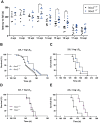
Prion infections cause neurodegeneration, which often goes along with oxidative stress. However, the cellular source of reactive oxygen species (ROS) and their pathogenetic significance are unclear. Here we analyzed the contribution of NOX2, a prominent NADPH oxidase, to prion diseases. We found that NOX2 is markedly upregulated in microglia within affected brain regions of patients with Creutzfeldt-Jakob disease (CJD). Similarly, NOX2 expression was upregulated in prion-inoculated mouse brains and in murine cerebellar organotypic cultured slices (COCS). We then removed microglia from COCS using a ganciclovir-dependent lineage ablation strategy. NOX2 became undetectable in ganciclovir-treated COCS, confirming its microglial origin. Upon challenge with prions, NOX2-deficient mice showed delayed onset of motor deficits and a modest, but significant prolongation of survival. Dihydroethidium assays demonstrated a conspicuous ROS burst at the terminal stage of disease in wild-type mice, but not in NOX2-ablated mice. Interestingly, the improved motor performance in NOX2 deficient mice was already measurable at earlier stages of the disease, between 13 and 16 weeks post-inoculation. We conclude that NOX2 is a major source of ROS in prion diseases and can affect prion pathogenesis.
Conflict of interest statement
AA was supported by the Novartis Research Foundation. This does not alter our adherence to all PLOS Pathogens policies on sharing data and materials.
Figures



Similar articles
-
Nox2-containing NADPH oxidase deficiency confers protection from hindlimb ischemia in conditions of increased oxidative stress.Arterioscler Thromb Vasc Biol. 2009 Oct;29(10):1522-8. doi: 10.1161/ATVBAHA.109.191437. Epub 2009 Jul 2. Arterioscler Thromb Vasc Biol. 2009. PMID: 19574557
-
Arterial hypertension in a murine model of sleep apnea: role of NADPH oxidase 2.J Hypertens. 2014 Feb;32(2):300-5. doi: 10.1097/HJH.0000000000000016. J Hypertens. 2014. PMID: 24270180
-
NADPH oxidase NOX2 mediates rapid cellular oxidation following ATP stimulation of endotoxin-primed macrophages.J Immunol. 2009 Sep 1;183(5):3302-8. doi: 10.4049/jimmunol.0900394. J Immunol. 2009. PMID: 19696433
-
Phagocyte NADPH oxidase and specific immunity.Clin Sci (Lond). 2015 May 1;128(10):635-48. doi: 10.1042/CS20140635. Clin Sci (Lond). 2015. PMID: 25760962 Review.
-
Prion diseases of humans and animals: their causes and molecular basis.Annu Rev Neurosci. 2001;24:519-50. doi: 10.1146/annurev.neuro.24.1.519. Annu Rev Neurosci. 2001. PMID: 11283320 Review.
Cited by
-
Apocynin, an NADPH Oxidase Enzyme Inhibitor, Prevents Amebic Liver Abscess in Hamster.Biomedicines. 2023 Aug 21;11(8):2322. doi: 10.3390/biomedicines11082322. Biomedicines. 2023. PMID: 37626818 Free PMC article.
-
Microglia in prion diseases.J Clin Invest. 2017 Sep 1;127(9):3230-3239. doi: 10.1172/JCI90605. Epub 2017 Jul 17. J Clin Invest. 2017. PMID: 28714865 Free PMC article. Review.
-
Region-specific glial homeostatic signature in prion diseases is replaced by a uniform neuroinflammation signature, common for brain regions and prion strains with different cell tropism.Neurobiol Dis. 2020 Apr;137:104783. doi: 10.1016/j.nbd.2020.104783. Epub 2020 Jan 27. Neurobiol Dis. 2020. PMID: 32001329 Free PMC article.
-
Modeling α-Synucleinopathy in Organotypic Brain Slice Culture with Preformed α-Synuclein Amyloid Fibrils.J Parkinsons Dis. 2020;10(4):1397-1410. doi: 10.3233/JPD-202026. J Parkinsons Dis. 2020. PMID: 32716318 Free PMC article.
-
Guidelines for the Detection of NADPH Oxidases by Immunoblot and RT-qPCR.Methods Mol Biol. 2019;1982:191-229. doi: 10.1007/978-1-4939-9424-3_12. Methods Mol Biol. 2019. PMID: 31172474 Free PMC article.
References
-
- Aguzzi A, Nuvolone M, Zhu C (2013) The immunobiology of prion diseases. Nat Rev Immunol 13: 888–902. - PubMed
-
- Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, et al. (1996) Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature 379: 339–343. - PubMed
-
- Mallucci G, Dickinson A, Linehan J, Klohn PC, Brandner S, et al. (2003) Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science 302: 871–874. - PubMed
-
- Bueler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, et al. (1993) Mice devoid of PrP are resistant to scrapie. Cell 73: 1339–1347. - PubMed
-
- Moreno JA, Halliday M, Molloy C, Radford H, Verity N, et al. (2013) Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci Transl Med 5: 206ra138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

