Spartan deficiency causes genomic instability and progeroid phenotypes
- PMID: 25501849
- PMCID: PMC4269170
- DOI: 10.1038/ncomms6744
Spartan deficiency causes genomic instability and progeroid phenotypes
Abstract
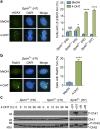
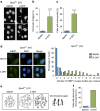
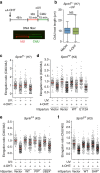
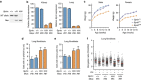
Spartan (also known as DVC1 and C1orf124) is a PCNA-interacting protein implicated in translesion synthesis, a DNA damage tolerance process that allows the DNA replication machinery to replicate past nucleotide lesions. However, the physiological relevance of Spartan has not been established. Here we report that Spartan insufficiency in mice causes chromosomal instability, cellular senescence and early onset of age-related phenotypes. Whereas complete loss of Spartan causes early embryonic lethality, hypomorphic mice with low amounts of Spartan are viable. These mice are growth retarded and develop cataracts, lordokyphosis and cachexia at a young age. Cre-mediated depletion of Spartan from conditional knockout mouse embryonic fibroblasts results in impaired lesion bypass, incomplete DNA replication, formation of micronuclei and chromatin bridges and eventually cell death. These data demonstrate that Spartan plays a key role in maintaining structural and numerical chromosome integrity and suggest a link between Spartan insufficiency and progeria.
Figures


Similar articles
-
Spartan deficiency causes accumulation of Topoisomerase 1 cleavage complexes and tumorigenesis.Nucleic Acids Res. 2017 May 5;45(8):4564-4576. doi: 10.1093/nar/gkx107. Nucleic Acids Res. 2017. PMID: 28199696 Free PMC article.
-
Defective ATM-Kap-1-mediated chromatin remodeling impairs DNA repair and accelerates senescence in progeria mouse model.Aging Cell. 2013 Apr;12(2):316-8. doi: 10.1111/acel.12035. Epub 2012 Dec 21. Aging Cell. 2013. PMID: 23173799
-
Spartan/C1orf124 is important to prevent UV-induced mutagenesis.Cell Cycle. 2012 Sep 15;11(18):3395-402. doi: 10.4161/cc.21694. Epub 2012 Aug 16. Cell Cycle. 2012. PMID: 22894931 Free PMC article.
-
Unraveling the mysteries of aging through a Hutchinson-Gilford progeria syndrome model.Rejuvenation Res. 2011 Apr;14(2):133-41. doi: 10.1089/rej.2010.1088. Epub 2011 Jan 5. Rejuvenation Res. 2011. PMID: 21208065 Review.
-
From the rarest to the most common: insights from progeroid syndromes into skin cancer and aging.J Invest Dermatol. 2009 Oct;129(10):2340-50. doi: 10.1038/jid.2009.103. Epub 2009 Apr 23. J Invest Dermatol. 2009. PMID: 19387478 Review.
Cited by
-
The identification of translesion DNA synthesis regulators: Inhibitors in the spotlight.DNA Repair (Amst). 2015 Aug;32:158-164. doi: 10.1016/j.dnarep.2015.04.027. Epub 2015 May 12. DNA Repair (Amst). 2015. PMID: 26002196 Free PMC article. Review.
-
Telomere Maintenance Mechanisms in a Cohort of High-Risk Neuroblastoma Tumors and Its Relation to Genomic Variants in the TERT and ATRX Genes.Cancers (Basel). 2023 Dec 7;15(24):5732. doi: 10.3390/cancers15245732. Cancers (Basel). 2023. PMID: 38136279 Free PMC article.
-
From the TOP: Formation, recognition and resolution of topoisomerase DNA protein crosslinks.DNA Repair (Amst). 2024 Oct;142:103751. doi: 10.1016/j.dnarep.2024.103751. Epub 2024 Aug 16. DNA Repair (Amst). 2024. PMID: 39180935 Review.
-
The intimate genetics of Drosophila fertilization.Open Biol. 2015 Aug;5(8):150076. doi: 10.1098/rsob.150076. Open Biol. 2015. PMID: 26246493 Free PMC article. Review.
-
DNA protein crosslink proteolysis repair: From yeast to premature ageing and cancer in humans.DNA Repair (Amst). 2018 Nov;71:198-204. doi: 10.1016/j.dnarep.2018.08.025. Epub 2018 Aug 23. DNA Repair (Amst). 2018. PMID: 30170832 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

