CXCR4 receptor overexpression in mesenchymal stem cells facilitates treatment of acute lung injury in rats
- PMID: 25492872
- PMCID: PMC4303655
- DOI: 10.1074/jbc.M114.605063
CXCR4 receptor overexpression in mesenchymal stem cells facilitates treatment of acute lung injury in rats
Abstract
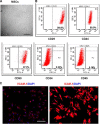
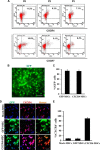
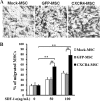
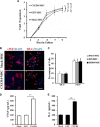
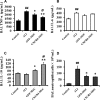
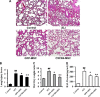
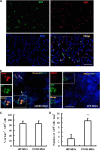
Novel therapeutic regimens for tissue renewal incorporate mesenchymal stem cells (MSCs) as they differentiate into a variety of cell types and are a stem cell type that is easy to harvest and to expand in vitro. However, surface chemokine receptors, such as CXCR4, which are involved in the mobilization of MSCs, are expressed only on the surface of a small proportion of MSCs, and the lack of CXCR4 expression may underlie the low efficiency of homing of MSCs toward tissue damage, which results in a poor curative effect. Here, a rat CXCR4 expressing lentiviral vector was constructed and introduced into MSCs freshly prepared from rat bone marrow. The influence of CXCR4 expression on migration, proliferation, differentiation, and paracrine effects of MSCs was examined in vitro. The in vivo properties of CXCR4-MSCs were also investigated in a model of acute lung injury in rats induced by lipopolysaccharide. Expression of CXCR4 in MSCs significantly enhanced the chemotactic and paracrine characteristics of the cells in vitro but did not affect self-renewal or differentiation into alveolar and vascular endothelial cells. In vivo, CXCR4 improved MSC homing and colonization of damaged lung tissue, and furthermore, the transplanted CXCR4-MSCs suppressed the development of acute lung injury in part by modulating levels of inflammatory molecules and the neutrophil count. These results indicated that efficient mobilization of MSCs to sites of tissue injury may be due to CXCR4, and therefore, increased expression of CXCR4 may improve their therapeutic potential in the treatment of diseases where tissue damage develops.
Keywords: Cell Migration; Lung Injury; Mesenchymal Stem Cells (MSCs); Migration; Transplantation.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Similar articles
-
Genetic Modification of Mesenchymal Stem Cells Overexpressing Angiotensin II Type 2 Receptor Increases Cell Migration to Injured Lung in LPS-Induced Acute Lung Injury Mice.Stem Cells Transl Med. 2018 Oct;7(10):721-730. doi: 10.1002/sctm.17-0279. Epub 2018 Aug 21. Stem Cells Transl Med. 2018. PMID: 30133167 Free PMC article.
-
Targeted migration of mesenchymal stem cells modified with CXCR4 to acute failing liver improves liver regeneration.World J Gastroenterol. 2014 Oct 28;20(40):14884-94. doi: 10.3748/wjg.v20.i40.14884. World J Gastroenterol. 2014. PMID: 25356048 Free PMC article.
-
Mesenchymal stem cells overexpressing C-X-C chemokine receptor type 4 improve early liver regeneration of small-for-size liver grafts.Liver Transpl. 2013 Feb;19(2):215-25. doi: 10.1002/lt.23577. Liver Transpl. 2013. PMID: 23193024
-
Enhancing the migration ability of mesenchymal stromal cells by targeting the SDF-1/CXCR4 axis.Biomed Res Int. 2013;2013:561098. doi: 10.1155/2013/561098. Epub 2013 Dec 5. Biomed Res Int. 2013. PMID: 24381939 Free PMC article. Review.
-
Hypoxic Preconditioning of Human Umbilical Cord Mesenchymal Stem Cells Is an Effective Strategy for Treating Acute Lung Injury.Stem Cells Dev. 2021 Feb;30(3):128-134. doi: 10.1089/scd.2020.0174. Epub 2021 Jan 18. Stem Cells Dev. 2021. PMID: 33349130 Review.
Cited by
-
Genetic Modification of Mesenchymal Stem Cells Overexpressing Angiotensin II Type 2 Receptor Increases Cell Migration to Injured Lung in LPS-Induced Acute Lung Injury Mice.Stem Cells Transl Med. 2018 Oct;7(10):721-730. doi: 10.1002/sctm.17-0279. Epub 2018 Aug 21. Stem Cells Transl Med. 2018. PMID: 30133167 Free PMC article.
-
Mesenchymal stem/stromal cell therapy for COVID-19 pneumonia: potential mechanisms, current clinical evidence, and future perspectives.Stem Cell Res Ther. 2022 Mar 24;13(1):124. doi: 10.1186/s13287-022-02810-6. Stem Cell Res Ther. 2022. PMID: 35321737 Free PMC article. Review.
-
Are the Properties of Bone Marrow-Derived Mesenchymal Stem Cells Influenced by Overweight and Obesity?Int J Mol Sci. 2023 Mar 2;24(5):4831. doi: 10.3390/ijms24054831. Int J Mol Sci. 2023. PMID: 36902259 Free PMC article. Review.
-
Current Status of Cell-Based Therapies for COVID-19: Evidence From Mesenchymal Stromal Cells in Sepsis and ARDS.Front Immunol. 2021 Oct 1;12:738697. doi: 10.3389/fimmu.2021.738697. eCollection 2021. Front Immunol. 2021. PMID: 34659231 Free PMC article. Review.
-
Mesenchymal stem cell therapy for acute respiratory distress syndrome: from basic to clinics.Protein Cell. 2020 Oct;11(10):707-722. doi: 10.1007/s13238-020-00738-2. Epub 2020 Jun 9. Protein Cell. 2020. PMID: 32519302 Free PMC article. Review.
References
-
- Dushianthan A., Grocott M. P., Postle A. D., Cusack R. (2011) Acute respiratory distress syndrome and acute lung injury. Postgrad. Med. J. 87, 612–622 - PubMed
-
- Villar J., Sulemanji D., Kacmarek R. M. (2014) The acute respiratory distress syndrome: incidence and mortality, has it changed? Curr. Opin. Crit. Care 20, 3–9 - PubMed
-
- Spieth P. M., Zhang H. (2014) Pharmacological therapies for acute respiratory distress syndrome. Curr. Opin. Crit. Care 20, 113–121 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

