Stress granules, P-bodies and cancer
- PMID: 25482014
- PMCID: PMC4457708
- DOI: 10.1016/j.bbagrm.2014.11.009
Stress granules, P-bodies and cancer
Abstract
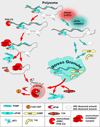
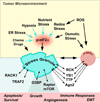
Cancer cells are exposed to adverse conditions in the tumor microenvironment, and utilize post-transcriptional control mechanisms to re-program gene expression in ways that enhance cell survival. Stress granules and processing bodies are RNA-containing granules that contribute to this process by modulating cellular signaling pathways, metabolic machinery, and stress response programs. This review examines evidence implicating RNA granules in the pathogenesis of cancer and discusses their potential as targets for anticancer therapies. This article is part of a Special Issue entitled: Translation and Cancer.
Keywords: Apoptosis; Cancer; P-body; Post-transcriptional regulation; Stress granule; Stress response.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Translational control of aberrant stress responses as a hallmark of cancer.J Pathol. 2018 Apr;244(5):650-666. doi: 10.1002/path.5030. Epub 2018 Feb 20. J Pathol. 2018. PMID: 29293271 Review.
-
The role of IRES trans-acting factors in carcinogenesis.Biochim Biophys Acta. 2015 Jul;1849(7):887-97. doi: 10.1016/j.bbagrm.2014.09.012. Epub 2014 Sep 23. Biochim Biophys Acta. 2015. PMID: 25257759 Review.
-
Stress-mediated translational control in cancer cells.Biochim Biophys Acta. 2015 Jul;1849(7):845-60. doi: 10.1016/j.bbagrm.2014.11.002. Epub 2014 Nov 10. Biochim Biophys Acta. 2015. PMID: 25464034 Review.
-
Translational control mechanisms in metabolic regulation: critical role of RNA binding proteins, microRNAs, and cytoplasmic RNA granules.Am J Physiol Endocrinol Metab. 2011 Dec;301(6):E1051-64. doi: 10.1152/ajpendo.00399.2011. Epub 2011 Oct 4. Am J Physiol Endocrinol Metab. 2011. PMID: 21971522 Review.
-
Cytoplasmic stress granules: Dynamic modulators of cell signaling and disease.Biochim Biophys Acta Mol Basis Dis. 2017 Apr;1863(4):884-895. doi: 10.1016/j.bbadis.2016.12.022. Epub 2017 Jan 15. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 28095315 Review.
Cited by
-
The function of RNA-binding proteins at the synapse: implications for neurodegeneration.Cell Mol Life Sci. 2015 Oct;72(19):3621-35. doi: 10.1007/s00018-015-1943-x. Epub 2015 Jun 6. Cell Mol Life Sci. 2015. PMID: 26047658 Free PMC article. Review.
-
Vinca alkaloid drugs promote stress-induced translational repression and stress granule formation.Oncotarget. 2016 May 24;7(21):30307-22. doi: 10.18632/oncotarget.8728. Oncotarget. 2016. PMID: 27083003 Free PMC article.
-
Pathophysiology of stress granules: An emerging link to diseases (Review).Int J Mol Med. 2022 Apr;49(4):44. doi: 10.3892/ijmm.2022.5099. Epub 2022 Feb 9. Int J Mol Med. 2022. PMID: 35137915 Free PMC article. Review.
-
Relocalization of Translation Termination and Ribosome Recycling Factors to Stress Granules Coincides with Elevated Stop-Codon Readthrough and Reinitiation Rates upon Oxidative Stress.Cells. 2023 Jan 8;12(2):259. doi: 10.3390/cells12020259. Cells. 2023. PMID: 36672194 Free PMC article.
-
Integrated stress response restricts macrophage necroptosis.Life Sci Alliance. 2021 Nov 11;5(1):e202101260. doi: 10.26508/lsa.202101260. Print 2022 Jan. Life Sci Alliance. 2021. PMID: 34764207 Free PMC article.
References
-
- Fasken MB, Corbett AH. Process or perish: quality control in mRNA biogenesis. Nat Struct Mol Biol. 2005;12:482–488. - PubMed
-
- Garneau NL, Wilusz J, Wilusz CJ. The highways and byways of mRNA decay. Nat Rev Mol Cell Biol. 2007;8:113–126. - PubMed
-
- Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

