Type I interferon contributes to noncanonical inflammasome activation, mediates immunopathology, and impairs protective immunity during fatal infection with lipopolysaccharide-negative ehrlichiae
- PMID: 25481711
- PMCID: PMC4305182
- DOI: 10.1016/j.ajpath.2014.10.005
Type I interferon contributes to noncanonical inflammasome activation, mediates immunopathology, and impairs protective immunity during fatal infection with lipopolysaccharide-negative ehrlichiae
Abstract
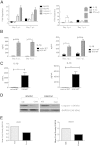
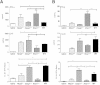
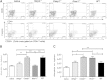
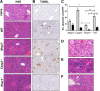
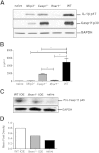
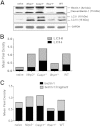
Ehrlichia species are intracellular bacteria that cause fatal ehrlichiosis, mimicking toxic shock syndrome in humans and mice. Virulent ehrlichiae induce inflammasome activation leading to caspase-1 cleavage and IL-18 secretion, which contribute to development of fatal ehrlichiosis. We show that fatal infection triggers expression of inflammasome components, activates caspase-1 and caspase-11, and induces host-cell death and secretion of IL-1β, IL-1α, and type I interferon (IFN-I). Wild-type and Casp1(-/-) mice were highly susceptible to fatal ehrlichiosis, had overwhelming infection, and developed extensive tissue injury. Nlrp3(-/-) mice effectively cleared ehrlichiae, but displayed acute mortality and developed liver injury similar to wild-type mice. By contrast, Ifnar1(-/-) mice were highly resistant to fatal disease and had lower bacterial burden, attenuated pathology, and prolonged survival. Ifnar1(-/-) mice also had improved protective immune responses mediated by IFN-γ and CD4(+) Th1 and natural killer T cells, with lower IL-10 secretion by T cells. Importantly, heightened resistance of Ifnar1(-/-) mice correlated with improved autophagosome processing, and attenuated noncanonical inflammasome activation indicated by decreased activation of caspase-11 and decreased IL-1β, compared with other groups. Our findings demonstrate that IFN-I signaling promotes host susceptibility to fatal ehrlichiosis, because it mediates ehrlichia-induced immunopathology and supports bacterial replication, perhaps via activation of noncanonical inflammasomes, reduced autophagy, and suppression of protective CD4(+) T cells and natural killer T-cell responses against ehrlichiae.
Copyright © 2015 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures





Similar articles
-
MyD88-dependent inflammasome activation and autophagy inhibition contributes to Ehrlichia-induced liver injury and toxic shock.PLoS Pathog. 2017 Oct 19;13(10):e1006644. doi: 10.1371/journal.ppat.1006644. eCollection 2017 Oct. PLoS Pathog. 2017. PMID: 29049365 Free PMC article.
-
Interferon Type I Regulates Inflammasome Activation and High Mobility Group Box 1 Translocation in Hepatocytes During Ehrlichia-Induced Acute Liver Injury.Hepatol Commun. 2020 Sep 27;5(1):33-51. doi: 10.1002/hep4.1608. eCollection 2021 Jan. Hepatol Commun. 2020. PMID: 33437899 Free PMC article.
-
Emerging Roles of Autophagy and Inflammasome in Ehrlichiosis.Front Immunol. 2019 May 8;10:1011. doi: 10.3389/fimmu.2019.01011. eCollection 2019. Front Immunol. 2019. PMID: 31134081 Free PMC article. Review.
-
Relative importance of T-cell subsets in monocytotropic ehrlichiosis: a novel effector mechanism involved in Ehrlichia-induced immunopathology in murine ehrlichiosis.Infect Immun. 2007 Sep;75(9):4608-20. doi: 10.1128/IAI.00198-07. Epub 2007 Jun 11. Infect Immun. 2007. PMID: 17562770 Free PMC article.
-
Non-Canonical Inflammasome Pathway: The Role of Cell Death and Inflammation in Ehrlichiosis.Cells. 2023 Nov 9;12(22):2597. doi: 10.3390/cells12222597. Cells. 2023. PMID: 37998332 Free PMC article. Review.
Cited by
-
The Prostaglandin E2-EP3 Receptor Axis Regulates Anaplasma phagocytophilum-Mediated NLRC4 Inflammasome Activation.PLoS Pathog. 2016 Aug 2;12(8):e1005803. doi: 10.1371/journal.ppat.1005803. eCollection 2016 Aug. PLoS Pathog. 2016. PMID: 27482714 Free PMC article.
-
MyD88-dependent inflammasome activation and autophagy inhibition contributes to Ehrlichia-induced liver injury and toxic shock.PLoS Pathog. 2017 Oct 19;13(10):e1006644. doi: 10.1371/journal.ppat.1006644. eCollection 2017 Oct. PLoS Pathog. 2017. PMID: 29049365 Free PMC article.
-
Interferon signalling and non-canonical inflammasome activation promote host protection against multidrug-resistant Acinetobacter baumannii.Commun Biol. 2024 Nov 12;7(1):1494. doi: 10.1038/s42003-024-07204-3. Commun Biol. 2024. PMID: 39533032 Free PMC article.
-
Regulation of Inflammatory Cell Death by Phosphorylation.Front Immunol. 2022 Mar 1;13:851169. doi: 10.3389/fimmu.2022.851169. eCollection 2022. Front Immunol. 2022. PMID: 35300338 Free PMC article. Review.
-
Epidemiologic, Clinical and Immunological Consequences of Co-Infections during Canine Leishmaniosis.Animals (Basel). 2021 Nov 10;11(11):3206. doi: 10.3390/ani11113206. Animals (Basel). 2021. PMID: 34827938 Free PMC article. Review.
References
-
- Olano J.P., Walker D.H. Human ehrlichioses. Med Clin North Am. 2002;86:375–392. - PubMed
-
- Walker D.H., Dumler J.S. Human monocytic and granulocytic ehrlichioses. Discovery and diagnosis of emerging tick-borne infections and the critical role of the pathologist. Arch Pathol Lab Med. 1997;121:785–791. - PubMed
-
- Fichtenbaum C.J., Peterson L.R., Weil G.J. Ehrlichiosis presenting as a life-threatening illness with features of the toxic shock syndrome. Am J Med. 1993;95:351–357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

