Inhibition of LSD1 reduces herpesvirus infection, shedding, and recurrence by promoting epigenetic suppression of viral genomes
- PMID: 25473037
- PMCID: PMC4416407
- DOI: 10.1126/scitranslmed.3010643
Inhibition of LSD1 reduces herpesvirus infection, shedding, and recurrence by promoting epigenetic suppression of viral genomes
Abstract
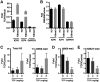
Herpesviruses are highly prevalent and maintain lifelong latent reservoirs, thus posing challenges to the control of herpetic disease despite the availability of antiviral pharmaceuticals that target viral DNA replication. The initiation of herpes simplex virus infection and reactivation from latency is dependent on a transcriptional coactivator complex that contains two required histone demethylases, LSD1 (lysine-specific demethylase 1) and a member of the JMJD2 family (Jumonji C domain-containing protein 2). Inhibition of either of these enzymes results in heterochromatic suppression of the viral genome and blocks infection and reactivation in vitro. We demonstrate that viral infection can be epigenetically suppressed in three animal models of herpes simplex virus infection and disease. Treating animals with the monoamine oxidase inhibitor tranylcypromine to inhibit LSD1 suppressed viral lytic infection, subclinical shedding, and reactivation from latency in vivo. This phenotypic suppression was correlated with enhanced epigenetic suppression of the viral genome and suggests that, even during latency, the chromatin state of the virus is dynamic. Therefore, epi-pharmaceuticals may represent a promising approach to treat herpetic diseases.
Copyright © 2014, American Association for the Advancement of Science.
Conflict of interest statement
Figures



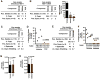
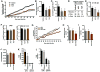
Similar articles
-
Herpesvirus latency.J Clin Invest. 2020 Jul 1;130(7):3361-3369. doi: 10.1172/JCI136225. J Clin Invest. 2020. PMID: 32364538 Free PMC article. Review.
-
A novel selective LSD1/KDM1A inhibitor epigenetically blocks herpes simplex virus lytic replication and reactivation from latency.mBio. 2013 Feb 5;4(1):e00558-12. doi: 10.1128/mBio.00558-12. mBio. 2013. PMID: 23386436 Free PMC article.
-
Inhibition of the histone demethylase LSD1 blocks alpha-herpesvirus lytic replication and reactivation from latency.Nat Med. 2009 Nov;15(11):1312-7. doi: 10.1038/nm.2051. Epub 2009 Oct 25. Nat Med. 2009. PMID: 19855399 Free PMC article.
-
Dynamic modulation of HSV chromatin drives initiation of infection and provides targets for epigenetic therapies.Virology. 2015 May;479-480:555-61. doi: 10.1016/j.virol.2015.01.026. Epub 2015 Feb 18. Virology. 2015. PMID: 25702087 Free PMC article. Review.
-
Targeting the JMJD2 histone demethylases to epigenetically control herpesvirus infection and reactivation from latency.Sci Transl Med. 2013 Jan 9;5(167):167ra5. doi: 10.1126/scitranslmed.3005145. Sci Transl Med. 2013. PMID: 23303604 Free PMC article.
Cited by
-
Transcriptional Elongation of HSV Immediate Early Genes by the Super Elongation Complex Drives Lytic Infection and Reactivation from Latency.Cell Host Microbe. 2017 Apr 12;21(4):507-517.e5. doi: 10.1016/j.chom.2017.03.007. Cell Host Microbe. 2017. PMID: 28407486 Free PMC article.
-
Histone demethylase LSD1 restricts influenza A virus infection by erasing IFITM3-K88 monomethylation.PLoS Pathog. 2017 Dec 27;13(12):e1006773. doi: 10.1371/journal.ppat.1006773. eCollection 2017 Dec. PLoS Pathog. 2017. PMID: 29281729 Free PMC article.
-
Herpesvirus latency.J Clin Invest. 2020 Jul 1;130(7):3361-3369. doi: 10.1172/JCI136225. J Clin Invest. 2020. PMID: 32364538 Free PMC article. Review.
-
Strategies that regulate LSD1 for novel therapeutics.Acta Pharm Sin B. 2024 Apr;14(4):1494-1507. doi: 10.1016/j.apsb.2024.01.005. Epub 2024 Jan 10. Acta Pharm Sin B. 2024. PMID: 38572094 Free PMC article. Review.
-
Inhibition of H3K27me3-specific histone demethylases JMJD3 and UTX blocks reactivation of herpes simplex virus 1 in trigeminal ganglion neurons.J Virol. 2015 Mar;89(6):3417-20. doi: 10.1128/JVI.03052-14. Epub 2014 Dec 31. J Virol. 2015. PMID: 25552720 Free PMC article.
References
-
- Whitley R, Kimberlin DW, Prober CG. In: Human Herpesviruses Biology, Therapy, and Immunoprophylaxis. Arvin A, Whitley R, editors. chap. 32. Cambridge University Press; Cambridge: 2007. pp. 589–601. - PubMed
-
- Roizman B, Knipe DM, Whitley RJ. In: Fields Virology. 6th. Knipe DM, Howley PM, editors. Lippincott Williams & Wilkins; Philadelphia: 2013.
-
- Kaye S, Choudhary A. Herpes simplex keratitis. Progress in retinal and eye research. 2006;25:355–380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

