Entrapment via synaptic-like connections between NG2 proteoglycan+ cells and dystrophic axons in the lesion plays a role in regeneration failure after spinal cord injury
- PMID: 25471575
- PMCID: PMC4252548
- DOI: 10.1523/JNEUROSCI.1309-14.2014
Entrapment via synaptic-like connections between NG2 proteoglycan+ cells and dystrophic axons in the lesion plays a role in regeneration failure after spinal cord injury
Abstract
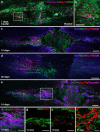
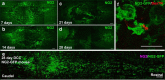
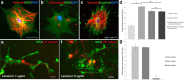
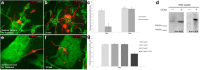
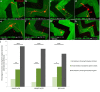
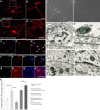
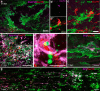
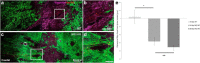
NG2 is purportedly one of the most growth-inhibitory chondroitin sulfate proteoglycans (CSPGs) produced after spinal cord injury. Nonetheless, once the severed axon tips dieback from the lesion core into the penumbra they closely associate with NG2+ cells. We asked if proteoglycans play a role in this tight cell-cell interaction and whether overadhesion upon these cells might participate in regeneration failure in rodents. Studies using varying ratios of CSPGs and adhesion molecules along with chondroitinase ABC, as well as purified adult cord-derived NG2 glia, demonstrate that CSPGs are involved in entrapping neurons. Once dystrophic axons become stabilized upon NG2+ cells, they form synaptic-like connections both in vitro and in vivo. In NG2 knock-out mice, sensory axons in the dorsal columns dieback further than their control counterparts. When axons are double conditioned to enhance their growth potential, some traverse the lesion core and express reduced amounts of synaptic proteins. Our studies suggest that proteoglycan-mediated entrapment upon NG2+ cells is an additional obstacle to CNS axon regeneration.
Keywords: NG2 proteoglycan; chondroitinase; dystrophic axons; glial scar; oligodendrocyte progenitor cells; spinal cord injury.
Copyright © 2014 the authors 0270-6474/14/3416369-16$15.00/0.
Figures

Similar articles
-
Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition.J Neurosci. 2003 Oct 15;23(28):9276-88. doi: 10.1523/JNEUROSCI.23-28-09276.2003. J Neurosci. 2003. PMID: 14561854 Free PMC article.
-
Adult NG2+ cells are permissive to neurite outgrowth and stabilize sensory axons during macrophage-induced axonal dieback after spinal cord injury.J Neurosci. 2010 Jan 6;30(1):255-65. doi: 10.1523/JNEUROSCI.3705-09.2010. J Neurosci. 2010. PMID: 20053907 Free PMC article.
-
Antibodies against the NG2 proteoglycan promote the regeneration of sensory axons within the dorsal columns of the spinal cord.J Neurosci. 2006 May 3;26(18):4729-39. doi: 10.1523/JNEUROSCI.3900-05.2006. J Neurosci. 2006. PMID: 16672645 Free PMC article.
-
NG2/CSPG4 and progranulin in the posttraumatic glial scar.Matrix Biol. 2018 Aug;68-69:571-588. doi: 10.1016/j.matbio.2017.10.002. Epub 2017 Oct 17. Matrix Biol. 2018. PMID: 29054751 Review.
-
Chondroitin sulfate proteoglycans: Key modulators in the developing and pathologic central nervous system.Exp Neurol. 2015 Jul;269:169-87. doi: 10.1016/j.expneurol.2015.04.006. Epub 2015 Apr 18. Exp Neurol. 2015. PMID: 25900055 Review.
Cited by
-
Olig2-Induced Semaphorin Expression Drives Corticospinal Axon Retraction After Spinal Cord Injury.Cereb Cortex. 2020 Oct 1;30(11):5702-5716. doi: 10.1093/cercor/bhaa142. Cereb Cortex. 2020. PMID: 32564090 Free PMC article.
-
Intravital imaging of axonal interactions with microglia and macrophages in a mouse dorsal column crush injury.J Vis Exp. 2014 Nov 23;(93):e52228. doi: 10.3791/52228. J Vis Exp. 2014. PMID: 25489963 Free PMC article.
-
STAT3 and SOCS3 regulate NG2 cell proliferation and differentiation after contusive spinal cord injury.Neurobiol Dis. 2016 May;89:10-22. doi: 10.1016/j.nbd.2016.01.017. Epub 2016 Jan 22. Neurobiol Dis. 2016. PMID: 26804026 Free PMC article.
-
In vivo reprogramming of NG2 glia enables adult neurogenesis and functional recovery following spinal cord injury.Cell Stem Cell. 2021 May 6;28(5):923-937.e4. doi: 10.1016/j.stem.2021.02.009. Epub 2021 Mar 5. Cell Stem Cell. 2021. PMID: 33675690 Free PMC article.
-
Understanding the axonal response to injury by in vivo imaging in the mouse spinal cord: A tale of two branches.Exp Neurol. 2019 Aug;318:277-285. doi: 10.1016/j.expneurol.2019.04.008. Epub 2019 Apr 12. Exp Neurol. 2019. PMID: 30986398 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
