Commensal microbiota is hepatoprotective and prevents liver fibrosis in mice
- PMID: 25466902
- PMCID: PMC4422368
- DOI: 10.1096/fj.14-259515
Commensal microbiota is hepatoprotective and prevents liver fibrosis in mice
Abstract
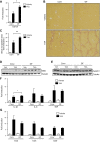
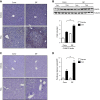
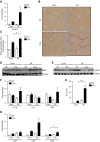
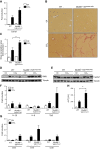
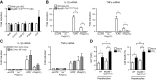
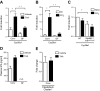
Translocation of bacteria and their products across the intestinal barrier is common in patients with liver disease, and there is evidence that experimental liver fibrosis depends on bacterial translocation. The purpose of our study was to investigate liver fibrosis in conventional and germ-free (GF) C57BL/6 mice. Chronic liver injury was induced by administration of thioacetamide (TAA) in the drinking water for 21 wk or by repeated intraperitoneal injections of carbon tetrachloride (CCl4). Increased liver fibrosis was observed in GF mice compared with conventional mice. Hepatocytes showed more toxin-induced oxidative stress and cell death. This was accompanied by increased activation of hepatic stellate cells, but hepatic mediators of inflammation were not significantly different. Similarly, a genetic model using Myd88/Trif-deficient mice, which lack downstream innate immunity signaling, had more severe fibrosis than wild-type mice. Isolated Myd88/Trif-deficient hepatocytes were more susceptible to toxin-induced cell death in culture. In conclusion, the commensal microbiota prevents fibrosis upon chronic liver injury in mice. This is the first study describing a beneficial role of the commensal microbiota in maintaining liver homeostasis and preventing liver fibrosis.
Keywords: bacterial translocation; innate immune system; microbiome.
© FASEB.
Figures
Similar articles
-
Involvement of the nuclear high mobility group B1 peptides released from injured hepatocytes in murine hepatic fibrogenesis.Biochim Biophys Acta. 2014 Sep;1842(9):1720-32. doi: 10.1016/j.bbadis.2014.06.017. Epub 2014 Jun 23. Biochim Biophys Acta. 2014. PMID: 24970745
-
Curcumin protects against thioacetamide-induced hepatic fibrosis by attenuating the inflammatory response and inducing apoptosis of damaged hepatocytes.J Nutr Biochem. 2012 Oct;23(10):1352-66. doi: 10.1016/j.jnutbio.2011.08.004. Epub 2012 Jan 4. J Nutr Biochem. 2012. PMID: 22221674
-
The role of CYP2A5 in liver injury and fibrosis: chemical-specific difference.Naunyn Schmiedebergs Arch Pharmacol. 2016 Jan;389(1):33-43. doi: 10.1007/s00210-015-1172-8. Epub 2015 Sep 12. Naunyn Schmiedebergs Arch Pharmacol. 2016. PMID: 26363552 Free PMC article.
-
Vitamin D inhibits proliferation and profibrotic marker expression in hepatic stellate cells and decreases thioacetamide-induced liver fibrosis in rats.Gut. 2011 Dec;60(12):1728-37. doi: 10.1136/gut.2010.234666. Epub 2011 Aug 4. Gut. 2011. PMID: 21816960
-
CD1d-restricted natural killer T cells contribute to hepatic inflammation and fibrogenesis in mice.J Hepatol. 2011 Jun;54(6):1195-204. doi: 10.1016/j.jhep.2010.08.022. Epub 2010 Nov 5. J Hepatol. 2011. PMID: 21145835
Cited by
-
Role of tenofovir dipivoxil in gut microbiota recovery from HBV-infection induced dysbiosis.BMC Microbiol. 2024 Sep 20;24(1):359. doi: 10.1186/s12866-024-03457-4. BMC Microbiol. 2024. PMID: 39304810 Free PMC article.
-
Microbiota and Fatty Liver Disease-the Known, the Unknown, and the Future.Cell Host Microbe. 2020 Aug 12;28(2):233-244. doi: 10.1016/j.chom.2020.07.007. Cell Host Microbe. 2020. PMID: 32791115 Free PMC article. Review.
-
Gut-Liver Axis and Non-Alcoholic Fatty Liver Disease: A Vicious Circle of Dysfunctions Orchestrated by the Gut Microbiome.Biology (Basel). 2022 Nov 6;11(11):1622. doi: 10.3390/biology11111622. Biology (Basel). 2022. PMID: 36358323 Free PMC article. Review.
-
Attenuated fibrosis in specific pathogen-free microbiota in experimental cholestasis- and toxin-induced liver injury.FASEB J. 2019 Nov;33(11):12464-12476. doi: 10.1096/fj.201901113R. Epub 2019 Aug 20. FASEB J. 2019. PMID: 31431085 Free PMC article.
-
Intestinal Microbiome-Macrophage Crosstalk Contributes to Cholestatic Liver Disease by Promoting Intestinal Permeability in Mice.Hepatology. 2020 Dec;72(6):2090-2108. doi: 10.1002/hep.31228. Hepatology. 2020. PMID: 32168395 Free PMC article.
References
-
- Schnabl B., Scholten D., Brenner D. A. (2008) What is the potential role of antifibrotic agents for the treatment of liver disease? Nat. Clin. Pract. Gastroenterol. Hepatol. 5, 496–497 - PubMed
-
- Pascual S., Such J., Esteban A., Zapater P., Casellas J. A., Aparicio J. R., Girona E., Gutiérrez A., Carnices F., Palazón J. M., Sola-Vera J., Perez-Mateo M. (2003) Intestinal permeability is increased in patients with advanced cirrhosis. Hepatogastroenterology 50, 1482–1486 - PubMed
-
- Francés R., Zapater P., González-Navajas J. M., Muñoz C., Caño R., Moreu R., Pascual S., Bellot P., Pérez-Mateo M., Such J. (2008) Bacterial DNA in patients with cirrhosis and noninfected ascites mimics the soluble immune response established in patients with spontaneous bacterial peritonitis. Hepatology 47, 978–985 - PubMed
-
- Lin R. S., Lee F. Y., Lee S. D., Tsai Y. T., Lin H. C., Lu R. H., Hsu W. C., Huang C. C., Wang S. S., Lo K. J. (1995) Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J. Hepatol. 22, 165–172 - PubMed
-
- Wigg A. J., Roberts-Thomson I. C., Dymock R. B., McCarthy P. J., Grose R. H., Cummins A. G. (2001) The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut 48, 206–211 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AA020703/AA/NIAAA NIH HHS/United States
- P40 OD010995/OD/NIH HHS/United States
- P30 DK034987/DK/NIDDK NIH HHS/United States
- U01-AA021856/AA/NIAAA NIH HHS/United States
- P42 ES010337/ES/NIEHS NIH HHS/United States
- P30 GM103331/GM/NIGMS NIH HHS/United States
- 2P42-ES010337/ES/NIEHS NIH HHS/United States
- U01 AA021856/AA/NIAAA NIH HHS/United States
- K08-DK081830/DK/NIDDK NIH HHS/United States
- P40 RR018603/RR/NCRR NIH HHS/United States
- K08 DK081830/DK/NIDDK NIH HHS/United States
- U24 DK097193/DK/NIDDK NIH HHS/United States
- 1U24DK097193/DK/NIDDK NIH HHS/United States
- P40RR018603/RR/NCRR NIH HHS/United States
- I01 BX002213/BX/BLRD VA/United States
- P30-DK34987/DK/NIDDK NIH HHS/United States
- R01-AA020703/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

