Prion-like polymerization as a signaling mechanism
- PMID: 25457352
- PMCID: PMC4429004
- DOI: 10.1016/j.it.2014.10.003
Prion-like polymerization as a signaling mechanism
Abstract
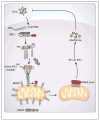
The innate immune system uses pattern recognition receptors such as RIG-I and NLRP3 to sense pathogen invasion and other danger signals. Activation of these receptors induces robust signal transduction cascades that trigger the production of cytokines important for host protection. MAVS and ASC are essential adaptor proteins downstream of RIG-I and NLRP3, respectively, and both contain N-terminal domains belonging to the death domain superfamily. Recent studies suggest that both MAVS and ASC form functional prion-like fibers through their respective death domains to propagate downstream signaling. Here, we review these findings, and in this context discuss the emerging concept of prion-like polymerization in signal transduction. We further examine the potential benefits of this signaling strategy, including signal amplification, host evolutionary advantage, and molecular memory.
Keywords: pattern recognition receptors; prion-like polymerization; signal transduction; signaling.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Prion-Like Polymerization in Immunity and Inflammation.Cold Spring Harb Perspect Biol. 2017 Apr 3;9(4):a023580. doi: 10.1101/cshperspect.a023580. Cold Spring Harb Perspect Biol. 2017. PMID: 27881448 Free PMC article. Review.
-
Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation.Cell. 2014 Mar 13;156(6):1207-1222. doi: 10.1016/j.cell.2014.01.063. Cell. 2014. PMID: 24630723 Free PMC article.
-
Mechanisms and pathways of innate immune activation and regulation in health and cancer.Hum Vaccin Immunother. 2014;10(11):3270-85. doi: 10.4161/21645515.2014.979640. Hum Vaccin Immunother. 2014. PMID: 25625930 Free PMC article. Review.
-
Accessory Factors of Cytoplasmic Viral RNA Sensors Required for Antiviral Innate Immune Response.Front Immunol. 2016 May 25;7:200. doi: 10.3389/fimmu.2016.00200. eCollection 2016. Front Immunol. 2016. PMID: 27252702 Free PMC article. Review.
-
Prions and Prion-like assemblies in neurodegeneration and immunity: The emergence of universal mechanisms across health and disease.Semin Cell Dev Biol. 2020 Mar;99:115-130. doi: 10.1016/j.semcdb.2019.11.012. Epub 2019 Dec 6. Semin Cell Dev Biol. 2020. PMID: 31818518 Review.
Cited by
-
Prion-like Aggregation of Mitochondrial Antiviral Signaling Protein in Lupus Patients Is Associated With Increased Levels of Type I Interferon.Arthritis Rheumatol. 2016 Nov;68(11):2697-2707. doi: 10.1002/art.39733. Arthritis Rheumatol. 2016. PMID: 27110677 Free PMC article.
-
Mechanism of TRIM25 Catalytic Activation in the Antiviral RIG-I Pathway.Cell Rep. 2016 Aug 2;16(5):1315-1325. doi: 10.1016/j.celrep.2016.06.070. Epub 2016 Jul 14. Cell Rep. 2016. PMID: 27425606 Free PMC article.
-
3β-hydroxysteroid-Δ24 reductase dampens anti-viral innate immune responses by targeting K27 ubiquitination of MAVS and STING.J Virol. 2023 Dec 21;97(12):e0151323. doi: 10.1128/jvi.01513-23. Epub 2023 Nov 30. J Virol. 2023. PMID: 38032198 Free PMC article.
-
Confocal Spectroscopy to Study Dimerization, Oligomerization and Aggregation of Proteins: A Practical Guide.Int J Mol Sci. 2016 Apr 30;17(5):655. doi: 10.3390/ijms17050655. Int J Mol Sci. 2016. PMID: 27144560 Free PMC article. Review.
-
Prions and Protein Assemblies that Convey Biological Information in Health and Disease.Neuron. 2016 Feb 3;89(3):433-48. doi: 10.1016/j.neuron.2016.01.026. Neuron. 2016. PMID: 26844828 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

