Angiotensin II limits NO production by upregulating arginase through a p38 MAPK-ATF-2 pathway
- PMID: 25446432
- PMCID: PMC4412038
- DOI: 10.1016/j.ejphar.2014.10.042
Angiotensin II limits NO production by upregulating arginase through a p38 MAPK-ATF-2 pathway
Abstract
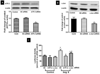
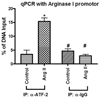
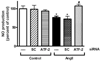
Enhanced vascular arginase activity can impair endothelium-dependent vasorelaxation by decreasing l-arginine availability to endothelial nitric oxide (NO) synthase, thereby reducing NO production and uncoupling NOS function. Elevated angiotensin II (Ang II) is a key component of endothelial dysfunction in many cardiovascular diseases and has been linked to elevated arginase activity. In this study we explored the signaling pathway leading to increased arginase expression/activity in response to Ang II in bovine aortic endothelial cells (BAEC). Our previous studies indicate involvement of p38 mitogen activated protein kinase (MAPK) in Ang II-induced arginase upregulation and reduced NO production. In this study, we further investigated the Ang II-transcriptional regulation of arginase 1 in endothelial cells. Our results indicate the involvement of ATF-2 transcription factor of the AP1 family in arginase 1 upregulation and in limiting NO production. Using small interfering RNA (siRNA) targeting ATF-2, we showed that this transcription factor is required for Ang II-induced arginase 1 gene upregulation and increased arginase 1 expression and activity, leading to reduced NO production. Electrophoretic mobility shift assay and chromatin immunoprecipitation assay further confirmed the involvement of ATF-2. Moreover, our data indicate that p38 MAPK phosphorylates ATF-2 in response to Ang II. Collectively, our results indicate that Ang II increases endothelial arginase activity/expression through a p38 MAPK/ATF-2 pathway leading to reduced endothelial NO production. These signaling steps might be therapeutic targets for preventing vascular endothelial dysfunction associated with elevated arginase activity/expression.
Keywords: ATF-2; Angiotensin II; Arginase; Nitric oxide (NO); Transcription factors; p38 MAPK.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures
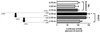
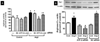



Similar articles
-
Angiotensin II-induced vascular endothelial dysfunction through RhoA/Rho kinase/p38 mitogen-activated protein kinase/arginase pathway.Am J Physiol Cell Physiol. 2011 May;300(5):C1181-92. doi: 10.1152/ajpcell.00328.2010. Epub 2011 Feb 2. Am J Physiol Cell Physiol. 2011. PMID: 21289285 Free PMC article.
-
Angiotensin II stimulates the synthesis of vascular endothelial growth factor through the p38 mitogen activated protein kinase pathway in cultured mouse podocytes.J Mol Endocrinol. 2006 Apr;36(2):377-88. doi: 10.1677/jme.1.02033. J Mol Endocrinol. 2006. PMID: 16595708
-
12-lipoxygenase pathway increases aldosterone production, 3',5'-cyclic adenosine monophosphate response element-binding protein phosphorylation, and p38 mitogen-activated protein kinase activation in H295R human adrenocortical cells.Endocrinology. 2003 Feb;144(2):534-43. doi: 10.1210/en.2002-220580. Endocrinology. 2003. PMID: 12538614
-
Angiotensin II: Role in oxidative stress, endothelial dysfunction, and diseases.Mol Cell Endocrinol. 2024 Oct 1;592:112309. doi: 10.1016/j.mce.2024.112309. Epub 2024 Jun 8. Mol Cell Endocrinol. 2024. PMID: 38852657 Review.
-
Inheritance and memory of stress-induced epigenome change: roles played by the ATF-2 family of transcription factors.Genes Cells. 2012 Apr;17(4):249-63. doi: 10.1111/j.1365-2443.2012.01587.x. Epub 2012 Mar 1. Genes Cells. 2012. PMID: 22380515 Free PMC article. Review.
Cited by
-
Tissue-specific up-regulation of arginase I and II induced by p38 MAPK mediates endothelial dysfunction in type 1 diabetes mellitus.Br J Pharmacol. 2015 Oct;172(19):4684-98. doi: 10.1111/bph.13242. Epub 2015 Aug 10. Br J Pharmacol. 2015. PMID: 26140333 Free PMC article.
-
Exacerbation of adverse cardiovascular effects of aircraft noise in an animal model of arterial hypertension.Redox Biol. 2020 Jul;34:101515. doi: 10.1016/j.redox.2020.101515. Epub 2020 Apr 18. Redox Biol. 2020. PMID: 32345536 Free PMC article.
-
Arginase: A Multifaceted Enzyme Important in Health and Disease.Physiol Rev. 2018 Apr 1;98(2):641-665. doi: 10.1152/physrev.00037.2016. Physiol Rev. 2018. PMID: 29412048 Free PMC article. Review.
-
Vascular surgical stretch injury leads to activation of P2X7 receptors and impaired endothelial function.PLoS One. 2017 Nov 14;12(11):e0188069. doi: 10.1371/journal.pone.0188069. eCollection 2017. PLoS One. 2017. PMID: 29136654 Free PMC article.
-
Normal Saline solutions cause endothelial dysfunction through loss of membrane integrity, ATP release, and inflammatory responses mediated by P2X7R/p38 MAPK/MK2 signaling pathways.PLoS One. 2019 Aug 14;14(8):e0220893. doi: 10.1371/journal.pone.0220893. eCollection 2019. PLoS One. 2019. PMID: 31412063 Free PMC article.
References
-
- Archer S. Measurement of nitric oxide in biological models. Faseb J. 1993;7:349–360. - PubMed
-
- Bachetti T, Comini L, Francolini G, Bastianon D, Valetti B, Cadei M, Grigolato P, Suzuki H, Finazzi D, Albertini A, Curello S, Ferrari R. Arginase pathway in human endothelial cells in pathophysiological conditions. J Mol Cell Cardiol. 2004;37:515–523. - PubMed
-
- Bagnost T, Berthelot A, Bouhaddi M, Laurant P, Andre C, Guillaume Y, Demougeot C. Treatment with the arginase inhibitor N(omega)-hydroxy-nor-L-arginine improves vascular function and lowers blood pressure in adult spontaneously hypertensive rat. Journal of hypertension. 2008;26:1110–1118. - PubMed
-
- Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare JM. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108:2000–2006. - PubMed
-
- Bivalacqua TJ, Burnett AL, Hellstrom WJ, Champion HC. Overexpression of arginase in the aged mouse penis impairs erectile function and decreases eNOS activity: influence of in vivo gene therapy of anti-arginase. Am J Physiol Heart Circ Physiol. 2007;292:H1340–1351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

