Interferon-stimulated gene 15 (ISG15) and ISG15-linked proteins can associate with members of the selective autophagic process, histone deacetylase 6 (HDAC6) and SQSTM1/p62
- PMID: 25429107
- PMCID: PMC4340396
- DOI: 10.1074/jbc.M114.593871
Interferon-stimulated gene 15 (ISG15) and ISG15-linked proteins can associate with members of the selective autophagic process, histone deacetylase 6 (HDAC6) and SQSTM1/p62
Abstract
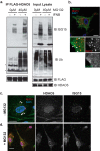
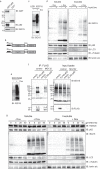
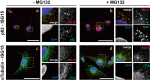
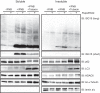
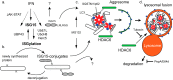
The ubiquitin-like interferon (IFN)-stimulated gene 15 (ISG15) and its specific E1, E2, and E3 enzymes are transcriptionally induced by type I IFNs. ISG15 conjugates newly synthesized proteins. ISG15 linkage to proteins appears to be an important downstream IFN signaling event that discriminates cellular and pathogenic proteins synthesized during IFN stimulation from existing proteins. This eliminates potentially pathogenic proteins as the cell attempts to return to normal homeostasis after IFN "stressed" conditions. However, the molecular events that occur in this process are not well known. Here, we show that the C-terminal LRLRGG of ISG15 interacts with the binder of ubiquitin zinc finger (BUZ) domain of histone deacetylase 6 (HDAC6). Because HDAC6 is involved in the autophagic clearance of ubiquitinated aggregates during which SQSTM1/p62 plays a major role as a cargo adapter, we also were able to confirm that p62 binds to ISG15 protein and its conjugated proteins upon forced expression. Both HDAC6 and p62 co-localized with ISG15 in an insoluble fraction of the cytosol, and this co-localization was magnified by the proteasome inhibitor MG132. In addition, ISG15 was degraded via the lysosome. Overexpression of ISG15, which leads to an increased conjugation level of the cellular proteome, enhanced autophagic degradation independently of IFN signaling transduction. These results thus indicate that ISG15 conjugation marks proteins for interaction with HDAC6 and p62 upon forced stressful conditions likely as a step toward autophagic clearance.
Keywords: Autophagy; Histone Deacetylase 6 (HDAC6); ISG15; Infection; Lysosome; Post-translational Modification (PTM); SQSTM1; Ubiquitin; Virus; p62.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
SQSTM1/p62 interacts with HDAC6 and regulates deacetylase activity.PLoS One. 2013 Sep 27;8(9):e76016. doi: 10.1371/journal.pone.0076016. eCollection 2013. PLoS One. 2013. PMID: 24086678 Free PMC article.
-
Modification of BECN1 by ISG15 plays a crucial role in autophagy regulation by type I IFN/interferon.Autophagy. 2015 Apr 3;11(4):617-28. doi: 10.1080/15548627.2015.1023982. Autophagy. 2015. PMID: 25906440 Free PMC article.
-
Regulation of MyD88 aggregation and the MyD88-dependent signaling pathway by sequestosome 1 and histone deacetylase 6.J Biol Chem. 2010 Nov 12;285(46):35759-69. doi: 10.1074/jbc.M110.126904. Epub 2010 Sep 13. J Biol Chem. 2010. PMID: 20837465 Free PMC article.
-
p62 links the autophagy pathway and the ubiqutin-proteasome system upon ubiquitinated protein degradation.Cell Mol Biol Lett. 2016 Dec 13;21:29. doi: 10.1186/s11658-016-0031-z. eCollection 2016. Cell Mol Biol Lett. 2016. PMID: 28536631 Free PMC article. Review.
-
The antiviral activities of ISG15.J Mol Biol. 2013 Dec 13;425(24):4995-5008. doi: 10.1016/j.jmb.2013.09.041. Epub 2013 Oct 3. J Mol Biol. 2013. PMID: 24095857 Free PMC article. Review.
Cited by
-
Attenuated clinical and osteoclastic phenotypes of Paget's disease of bone linked to the p.Pro392Leu/SQSTM1 mutation by a rare variant in the DOCK6 gene.BMC Med Genomics. 2022 Mar 3;15(1):41. doi: 10.1186/s12920-022-01198-9. BMC Med Genomics. 2022. PMID: 35241069 Free PMC article.
-
Modeling tumor immunity of mouse glioblastoma by exhausted CD8+ T cells.Sci Rep. 2018 Jan 9;8(1):208. doi: 10.1038/s41598-017-18540-2. Sci Rep. 2018. PMID: 29317703 Free PMC article.
-
Ubiquitin signaling and autophagy.J Biol Chem. 2018 Apr 13;293(15):5404-5413. doi: 10.1074/jbc.TM117.000117. Epub 2017 Nov 29. J Biol Chem. 2018. PMID: 29187595 Free PMC article. Review.
-
Post-translational Control of Innate Immune Signaling Pathways by Herpesviruses.Front Microbiol. 2019 Nov 14;10:2647. doi: 10.3389/fmicb.2019.02647. eCollection 2019. Front Microbiol. 2019. PMID: 31798565 Free PMC article. Review.
-
Epigenetic Regulation of Autophagy: A Path to the Control of Autoimmunity.Front Immunol. 2018 Aug 14;9:1864. doi: 10.3389/fimmu.2018.01864. eCollection 2018. Front Immunol. 2018. PMID: 30154791 Free PMC article. Review.
References
-
- Malakhov M. P., Malakhova O. A., Kim K. I., Ritchie K. J., Zhang D. E. (2002) UBP43 (USP18) specifically removes ISG15 from conjugated proteins. J. Biol. Chem. 277, 9976–9981 - PubMed
-
- Romijn R. A., Westein E., Bouma B., Schiphorst M. E., Sixma J. J., Lenting P. J., Huizinga E. G. (2003) Mapping the collagen-binding site in the von Willebrand factor-A3 domain. J. Biol. Chem. 278, 15035–15039 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

