Enhanced activation of an amino-terminally truncated isoform of the voltage-gated proton channel HVCN1 enriched in malignant B cells
- PMID: 25425665
- PMCID: PMC4273330
- DOI: 10.1073/pnas.1411390111
Enhanced activation of an amino-terminally truncated isoform of the voltage-gated proton channel HVCN1 enriched in malignant B cells
Abstract
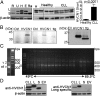
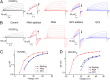
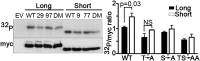
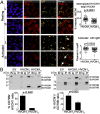
HVCN1 (Hydrogen voltage-gated channel 1) is the only mammalian voltage-gated proton channel. In human B lymphocytes, HVCN1 associates with the B-cell receptor (BCR) and is required for optimal BCR signaling and redox control. HVCN1 is expressed in malignant B cells that rely on BCR signaling, such as chronic lymphocytic leukemia (CLL) cells. However, little is known about its regulation in these cells. We found that HVCN1 was expressed in B cells as two protein isoforms. The shorter isoform (HVCN1S) was enriched in B cells from a cohort of 76 CLL patients. When overexpressed in a B-cell lymphoma line, HVCN1S responded more profoundly to protein kinase C-dependent phosphorylation. This more potent enhanced gating response was mediated by increased phosphorylation of the same residue responsible for enhanced gating in HVCN1L, Thr(29). Furthermore, the association of HVCN1S with the BCR was weaker, which resulted in its diminished internalization upon BCR stimulation. Finally, HVCN1S conferred a proliferative and migratory advantage as well as enhanced BCR-dependent signaling. Overall, our data show for the first time, to our knowledge, the existence of a shorter isoform of HVCN1 with enhanced gating that is specifically enriched in malignant B cells. The properties of HVCN1S suggest that it may contribute to the pathogenesis of BCR-dependent B-cell malignancies.
Keywords: Hv1; chronic lymphocytic leukemia; gating kinetics; phosphorylation; proton currents.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
HVCN1 modulates BCR signal strength via regulation of BCR-dependent generation of reactive oxygen species.Nat Immunol. 2010 Mar;11(3):265-72. doi: 10.1038/ni.1843. Epub 2010 Feb 7. Nat Immunol. 2010. PMID: 20139987 Free PMC article.
-
Identification of Thr29 as a critical phosphorylation site that activates the human proton channel Hvcn1 in leukocytes.J Biol Chem. 2010 Feb 19;285(8):5117-21. doi: 10.1074/jbc.C109.082727. Epub 2009 Dec 26. J Biol Chem. 2010. PMID: 20037153 Free PMC article.
-
Proton channel HVCN1 is required for effector functions of mouse eosinophils.BMC Immunol. 2013 May 24;14:24. doi: 10.1186/1471-2172-14-24. BMC Immunol. 2013. PMID: 23705768 Free PMC article.
-
Proteomic analysis of B-cell malignancies.J Proteomics. 2010 Sep 10;73(10):1804-22. doi: 10.1016/j.jprot.2010.03.010. Epub 2010 Mar 24. J Proteomics. 2010. PMID: 20346427 Review.
-
pH regulation and beyond: unanticipated functions for the voltage-gated proton channel, HVCN1.Trends Cell Biol. 2011 Jan;21(1):20-8. doi: 10.1016/j.tcb.2010.09.006. Epub 2010 Oct 18. Trends Cell Biol. 2011. PMID: 20961760 Free PMC article. Review.
Cited by
-
Altered pathways and targeted therapy in double hit lymphoma.J Hematol Oncol. 2022 Mar 18;15(1):26. doi: 10.1186/s13045-022-01249-9. J Hematol Oncol. 2022. PMID: 35303910 Free PMC article. Review.
-
Role of voltage-gated proton channel (Hv1) in cancer biology.Front Pharmacol. 2023 Apr 20;14:1175702. doi: 10.3389/fphar.2023.1175702. eCollection 2023. Front Pharmacol. 2023. PMID: 37153807 Free PMC article. Review.
-
Proton channels and renal hypertensive injury: a key piece of the Dahl salt-sensitive rat puzzle?Am J Physiol Regul Integr Comp Physiol. 2016 Apr 15;310(8):R679-90. doi: 10.1152/ajpregu.00115.2015. Epub 2016 Feb 3. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 26843580 Free PMC article. Review.
-
Unexpected expansion of the voltage-gated proton channel family.FEBS J. 2023 Feb;290(4):1008-1026. doi: 10.1111/febs.16617. Epub 2022 Sep 20. FEBS J. 2023. PMID: 36062330 Free PMC article.
-
Uncoupling histone modification crosstalk by engineering lysine demethylase LSD1.Nat Chem Biol. 2025 Feb;21(2):227-237. doi: 10.1038/s41589-024-01671-9. Epub 2024 Jul 4. Nat Chem Biol. 2025. PMID: 38965385
References
-
- Stevenson FK, Krysov S, Davies AJ, Steele AJ, Packham G. B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2011;118(16):4313–4320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

