Myeloid cell TRAF3 regulates immune responses and inhibits inflammation and tumor development in mice
- PMID: 25422508
- PMCID: PMC4272913
- DOI: 10.4049/jimmunol.1401548
Myeloid cell TRAF3 regulates immune responses and inhibits inflammation and tumor development in mice
Abstract
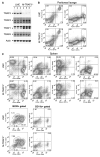
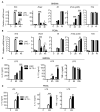
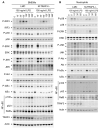
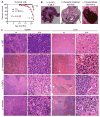
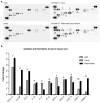
Myeloid cells, including granulocytes, monocytes, macrophages, and dendritic cells, are crucial players in innate immunity and inflammation. These cells constitutively or inducibly express a number of receptors of the TNFR and TLR families, whose signals are transduced by TNFR-associated factor (TRAF) molecules. In vitro studies showed that TRAF3 is required for TLR-induced type I IFN production, but the in vivo function of TRAF3 in myeloid cells remains unknown. In this article, we report the generation and characterization of myeloid cell-specific TRAF3-deficient (M-TRAF3(-/-)) mice, which allowed us to gain insights into the in vivo functions of TRAF3 in myeloid cells. We found that TRAF3 ablation did not affect the maturation or homeostasis of myeloid cells in young adult mice, even though TRAF3-deficient macrophages and neutrophils exhibited constitutive NF-κB2 activation. However, in response to injections with LPS (a bacterial mimic) or polyinosinic-polycytidylic acid (a viral mimic), M-TRAF3(-/-) mice exhibited an altered profile of cytokine production. M-TRAF3(-/-) mice immunized with T cell-independent and -dependent Ags displayed elevated T cell-independent IgG3 and T cell-dependent IgG2b responses. Interestingly, 15- to 22-mo-old M-TRAF3(-/-) mice spontaneously developed chronic inflammation or tumors, often affecting multiple organs. Taken together, our findings indicate that TRAF3 expressed in myeloid cells regulates immune responses in myeloid cells and acts to inhibit inflammation and tumor development in mice.
Copyright © 2014 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures
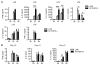


Similar articles
-
TNF receptor-associated factor 3 is required for T cell-mediated immunity and TCR/CD28 signaling.J Immunol. 2011 Jan 1;186(1):143-55. doi: 10.4049/jimmunol.1000290. Epub 2010 Nov 17. J Immunol. 2011. PMID: 21084666 Free PMC article.
-
Enhanced Toll-like receptor (TLR) responses of TNFR-associated factor 3 (TRAF3)-deficient B lymphocytes.J Leukoc Biol. 2011 Dec;90(6):1149-57. doi: 10.1189/jlb.0111044. Epub 2011 Oct 4. J Leukoc Biol. 2011. PMID: 21971520 Free PMC article.
-
Rescue of TRAF3-null mice by p100 NF-kappa B deficiency.J Exp Med. 2006 Oct 30;203(11):2413-8. doi: 10.1084/jem.20061166. Epub 2006 Oct 2. J Exp Med. 2006. PMID: 17015635 Free PMC article.
-
Roles for TNF-receptor associated factor 3 (TRAF3) in lymphocyte functions.Cytokine Growth Factor Rev. 2014 Apr;25(2):147-56. doi: 10.1016/j.cytogfr.2013.12.002. Epub 2013 Dec 25. Cytokine Growth Factor Rev. 2014. PMID: 24433987 Free PMC article. Review.
-
Roles of tumor necrosis factor receptor associated factor 3 (TRAF3) and TRAF5 in immune cell functions.Immunol Rev. 2011 Nov;244(1):55-74. doi: 10.1111/j.1600-065X.2011.01055.x. Immunol Rev. 2011. PMID: 22017431 Free PMC article. Review.
Cited by
-
TRAF3 deficiency promotes metabolic reprogramming in B cells.Sci Rep. 2016 Oct 18;6:35349. doi: 10.1038/srep35349. Sci Rep. 2016. PMID: 27752131 Free PMC article.
-
Extracellular fibrinogen-binding protein released by intracellular Staphylococcus aureus suppresses host immunity by targeting TRAF3.Nat Commun. 2022 Sep 19;13(1):5493. doi: 10.1038/s41467-022-33205-z. Nat Commun. 2022. PMID: 36123338 Free PMC article.
-
The impact of rumen-protected amino acids on the expression of key- genes involved in the innate immunity of dairy sheep.PLoS One. 2020 May 14;15(5):e0233192. doi: 10.1371/journal.pone.0233192. eCollection 2020. PLoS One. 2020. PMID: 32407360 Free PMC article.
-
Emerging Roles for Noncanonical NF-κB Signaling in the Modulation of Inflammatory Bowel Disease Pathobiology.Inflamm Bowel Dis. 2016 Sep;22(9):2265-79. doi: 10.1097/MIB.0000000000000858. Inflamm Bowel Dis. 2016. PMID: 27508514 Free PMC article. Review.
-
Characterization of Thymus-dependent and Thymus-independent Immunoglobulin Isotype Responses in Mice Using Enzyme-linked Immunosorbent Assay.J Vis Exp. 2018 Sep 7;(139):57843. doi: 10.3791/57843. J Vis Exp. 2018. PMID: 30247482 Free PMC article.
References
-
- Hacker H, Tseng PH, Karin M. Expanding TRAF function: TRAF3 as a tri-faced immune regulator. Nat Rev Immunol. 2011;11:457–468. - PubMed
-
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. - PubMed
-
- Saleh M. The machinery of Nod-like receptors: refining the paths to immunity and cell death. Immunol Rev. 2011;243:235–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

