Response gene to complement 32 (RGC-32) expression on M2-polarized and tumor-associated macrophages is M-CSF-dependent and enhanced by tumor-derived IL-4
- PMID: 25418473
- PMCID: PMC4716617
- DOI: 10.1038/cmi.2014.108
Response gene to complement 32 (RGC-32) expression on M2-polarized and tumor-associated macrophages is M-CSF-dependent and enhanced by tumor-derived IL-4
Abstract
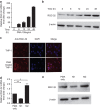
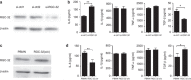
Response gene to complement 32 (RGC-32) is a cell cycle regulator involved in the proliferation, differentiation and migration of cells and has also been implicated in angiogenesis. Here we show that RGC-32 expression in macrophages is induced by IL-4 and reduced by LPS, indicating a link between RGC-32 expression and M2 polarization. We demonstrated that the increased expression of RGC-32 is characteristic of alternatively activated macrophages, in which this protein suppresses the production of pro-inflammatory cytokine IL-6 and promotes the production of the anti-inflammatory mediator TGF-β. Consistent with in vitro data, tumor-associated macrophages (TAMs) express high levels of RGC-32, and this expression is induced by tumor-derived ascitic fluid in an M-CSF- and/or IL-4-dependent manner. Collectively, these results establish RGC-32 as a marker for M2 macrophage polarization and indicate that this protein is a potential target for cancer immunotherapy, targeting tumor-associated macrophages.
Figures
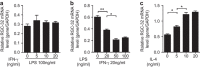

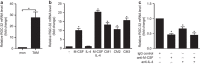
Comment in
-
Altering macrophage polarization in the tumor environment: the role of response gene to complement 32.Cell Mol Immunol. 2015 Nov;12(6):783-4. doi: 10.1038/cmi.2014.115. Epub 2014 Nov 24. Cell Mol Immunol. 2015. PMID: 25418471 Free PMC article. No abstract available.
Similar articles
-
Altering macrophage polarization in the tumor environment: the role of response gene to complement 32.Cell Mol Immunol. 2015 Nov;12(6):783-4. doi: 10.1038/cmi.2014.115. Epub 2014 Nov 24. Cell Mol Immunol. 2015. PMID: 25418471 Free PMC article. No abstract available.
-
Response gene to complement 32 expression in macrophages augments paracrine stimulation-mediated colon cancer progression.Cell Death Dis. 2019 Oct 10;10(10):776. doi: 10.1038/s41419-019-2006-2. Cell Death Dis. 2019. PMID: 31601783 Free PMC article.
-
Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin.Cell Immunol. 2013 Jan;281(1):51-61. doi: 10.1016/j.cellimm.2013.01.010. Epub 2013 Feb 4. Cell Immunol. 2013. PMID: 23454681
-
RGC-32 and diseases: the first 20 years.Immunol Res. 2019 Jun;67(2-3):267-279. doi: 10.1007/s12026-019-09080-0. Immunol Res. 2019. PMID: 31250246 Review.
-
Inflammatory cells and cancer: think different!J Exp Med. 2001 Mar 19;193(6):F23-6. doi: 10.1084/jem.193.6.f23. J Exp Med. 2001. PMID: 11257144 Free PMC article. Review. No abstract available.
Cited by
-
Response Gene to Complement 32 in Vascular Diseases.Front Cardiovasc Med. 2018 Sep 18;5:128. doi: 10.3389/fcvm.2018.00128. eCollection 2018. Front Cardiovasc Med. 2018. PMID: 30280101 Free PMC article. Review.
-
Reprogramming the tumor microenvironment by genome editing for precision cancer therapy.Mol Cancer. 2022 Apr 11;21(1):98. doi: 10.1186/s12943-022-01561-5. Mol Cancer. 2022. PMID: 35410257 Free PMC article. Review.
-
Accelerated Partial Breast Irradiation: Macrophage Polarisation Shift Classification Identifies High-Risk Tumours in Early Hormone Receptor-Positive Breast Cancer.Cancers (Basel). 2020 Feb 14;12(2):446. doi: 10.3390/cancers12020446. Cancers (Basel). 2020. PMID: 32075091 Free PMC article.
-
Regulator of Cell Cycle (RGCC) Expression During the Progression of Alzheimer's Disease.Cell Transplant. 2017 Apr 13;26(4):693-702. doi: 10.3727/096368916X694184. Epub 2016 Nov 30. Cell Transplant. 2017. PMID: 27938491 Free PMC article.
-
Tumor-associated macrophages: from basic research to clinical application.J Hematol Oncol. 2017 Feb 28;10(1):58. doi: 10.1186/s13045-017-0430-2. J Hematol Oncol. 2017. PMID: 28241846 Free PMC article. Review.
References
-
- 1Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol 2009; 27: 669–692. - PubMed
-
- 4Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 2006; 177: 7303–7311. - PubMed
-
- 5Locati M, Mantovani A, Sica A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv Immunol 2013; 120: 163–184. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

