Three RNA binding proteins form a complex to promote differentiation of germline stem cell lineage in Drosophila
- PMID: 25412508
- PMCID: PMC4238977
- DOI: 10.1371/journal.pgen.1004797
Three RNA binding proteins form a complex to promote differentiation of germline stem cell lineage in Drosophila
Abstract
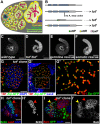
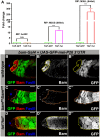
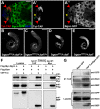
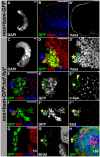
In regenerative tissues, one of the strategies to protect stem cells from genetic aberrations, potentially caused by frequent cell division, is to transiently expand the stem cell daughters before further differentiation. However, failure to exit the transit amplification may lead to overgrowth, and the molecular mechanism governing this regulation remains vague. In a Drosophila mutagenesis screen for factors involved in the regulation of germline stem cell (GSC) lineage, we isolated a mutation in the gene CG32364, which encodes a putative RNA-binding protein (RBP) and is designated as tumorous testis (tut). In tut mutant, spermatogonia fail to differentiate and over-amplify, a phenotype similar to that in mei-P26 mutant. Mei-P26 is a TRIM-NHL tumor suppressor homolog required for the differentiation of GSC lineage. We found that Tut binds preferentially a long isoform of mei-P26 3'UTR, and is essential for the translational repression of mei-P26 reporter. Bam and Bgcn are both RBPs that have also been shown to repress mei-P26 expression. Our genetic analyses indicate that tut, bam, or bgcn is required to repress mei-P26 and to promote the differentiation of GSCs. Biochemically, we demonstrate that Tut, Bam, and Bgcn can form a physical complex in which Bam holds Tut on its N-terminus and Bgcn on its C-terminus. Our in vivo and in vitro evidence illustrate that Tut acts with Bam, Bgcn to accurately coordinate proliferation and differentiation in Drosophila germline stem cell lineage.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
A self-limiting switch based on translational control regulates the transition from proliferation to differentiation in an adult stem cell lineage.Cell Stem Cell. 2012 Nov 2;11(5):689-700. doi: 10.1016/j.stem.2012.08.012. Cell Stem Cell. 2012. PMID: 23122292 Free PMC article.
-
Mei-p26 cooperates with Bam, Bgcn and Sxl to promote early germline development in the Drosophila ovary.PLoS One. 2013;8(3):e58301. doi: 10.1371/journal.pone.0058301. Epub 2013 Mar 20. PLoS One. 2013. PMID: 23526974 Free PMC article.
-
Bam and Bgcn in Drosophila germline stem cell differentiation.Vitam Horm. 2011;87:399-416. doi: 10.1016/B978-0-12-386015-6.00038-X. Vitam Horm. 2011. PMID: 22127253 Review.
-
Histone H3K9 trimethylase Eggless controls germline stem cell maintenance and differentiation.PLoS Genet. 2011 Dec;7(12):e1002426. doi: 10.1371/journal.pgen.1002426. Epub 2011 Dec 22. PLoS Genet. 2011. PMID: 22216012 Free PMC article.
-
Proliferative control in Drosophila stem cells.Curr Opin Cell Biol. 2008 Dec;20(6):699-706. doi: 10.1016/j.ceb.2008.10.002. Epub 2008 Nov 25. Curr Opin Cell Biol. 2008. PMID: 18996190 Free PMC article. Review.
Cited by
-
Functional Divergence of the bag-of-marbles Gene in the Drosophila melanogaster Species Group.Mol Biol Evol. 2022 Jul 2;39(7):msac137. doi: 10.1093/molbev/msac137. Mol Biol Evol. 2022. PMID: 35714266 Free PMC article.
-
MiMIC analysis reveals an isoform specific role for Drosophila Musashi in follicle stem cell maintenance and escort cell function.Cell Death Discov. 2022 Nov 12;8(1):455. doi: 10.1038/s41420-022-01245-5. Cell Death Discov. 2022. PMID: 36371343 Free PMC article.
-
YTHDC2 serves a distinct late role in spermatocytes during germ cell differentiation.Proc Natl Acad Sci U S A. 2024 Oct 15;121(42):e2309548121. doi: 10.1073/pnas.2309548121. Epub 2024 Oct 8. Proc Natl Acad Sci U S A. 2024. PMID: 39378093
-
Mettl1-dependent m7G tRNA modification is essential for maintaining spermatogenesis and fertility in Drosophila melanogaster.Nat Commun. 2024 Sep 24;15(1):8147. doi: 10.1038/s41467-024-52389-0. Nat Commun. 2024. PMID: 39317727 Free PMC article.
-
Srlp is crucial for the self-renewal and differentiation of germline stem cells via RpL6 signals in Drosophila testes.Cell Death Dis. 2019 Apr 1;10(4):294. doi: 10.1038/s41419-019-1527-z. Cell Death Dis. 2019. PMID: 30931935 Free PMC article.
References
-
- Alonso L, Fuchs E (2003) Stem cells in the skin: waste not, Wnt not. Genes & Development 17: 1189–1200. - PubMed
-
- Clarke MF, Fuller M (2006) Stem cells and cancer: Two faces of eve. Cell 124: 1111–1115. - PubMed
-
- Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, et al. (2003) Biology of hematopoietic stem cells and progenitors: Implications for clinical application. Annual Review of Immunology 21: 759–806. - PubMed
-
- Jamieson CHM, Ailles LE, Dylla SJ, Muijtjens M, Jones C, et al. (2004) Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. New England Journal of Medicine 351: 657–667. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

