MicroRNA-194 reciprocally stimulates osteogenesis and inhibits adipogenesis via regulating COUP-TFII expression
- PMID: 25412310
- PMCID: PMC4260743
- DOI: 10.1038/cddis.2014.485
MicroRNA-194 reciprocally stimulates osteogenesis and inhibits adipogenesis via regulating COUP-TFII expression
Abstract
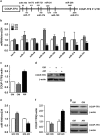
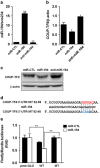
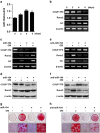
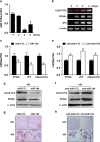
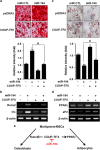
Osteoblasts and adipocytes are differentiated from common mesenchymal stem cells (MSCs) in processes which are tightly controlled by various growth factors, signaling molecules, transcriptional factors and microRNAs. Recently, chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) was identified as a critical regulator of MSC fate. In the present study, we aimed to identify some microRNAs (miR), which target COUP-TFII, and to determine the effects on MSCs fate. During osteoblastic or adipocytic differentiation from MSCs lineage cells, miR-194 expression was found to be reversal. In the cultures of mesenchymal C3H10T1/2 and primary bone marrow stromal cells, osteogenic stimuli increased miR-194 expression with accompanying decreases in COUP-TFII expression, whereas adipogenic stimuli reduced miR-194 expression with accompanying increases in COUP-TFII expression. A luciferase assay with COUP-TFII 3'-untranslated region (UTR) reporter plasmid, including the miR-194 binding sequences, showed that the introduction of miR-194 reduced the luciferase activity. However, it did not affect the activity of mutated COUP-TFII 3'-UTR reporter. Enforced expression of miR-194 significantly enhanced osteoblast differentiation, but inhibited adipocyte differentiation by decreasing COUP-TFII mRNA and protein levels. In contrast, inhibition of the endogenous miR-194 reduced matrix mineralization in the MSCs cultures, promoting the formation of lipid droplets by rescuing COUP-TFII expression. Furthermore, overexpression of COUP-TFII reversed the effects of miR-194 on the cell fates. Taken together, our results showed that miR-194 acts as a critical regulator of COUP-TFII, and can determinate the fate of MSCs to differentiate into osteoblasts and adipocytes. This suggests that miR-194 and COUP-TFII may be good target molecules for controlling bone and metabolic diseases.
Figures
Similar articles
-
MicroRNA-302a stimulates osteoblastic differentiation by repressing COUP-TFII expression.J Cell Physiol. 2015 Apr;230(4):911-21. doi: 10.1002/jcp.24822. J Cell Physiol. 2015. PMID: 25215426
-
Orphan nuclear receptor chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) protein negatively regulates bone morphogenetic protein 2-induced osteoblast differentiation through suppressing runt-related gene 2 (Runx2) activity.J Biol Chem. 2012 Jun 1;287(23):18888-99. doi: 10.1074/jbc.M111.311878. Epub 2012 Apr 6. J Biol Chem. 2012. PMID: 22493443 Free PMC article.
-
Nuclear receptor chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) modulates mesenchymal cell commitment and differentiation.Proc Natl Acad Sci U S A. 2011 Sep 6;108(36):14843-8. doi: 10.1073/pnas.1110236108. Epub 2011 Aug 22. Proc Natl Acad Sci U S A. 2011. PMID: 21873211 Free PMC article.
-
The role of microRNAs in cell fate determination of mesenchymal stem cells: balancing adipogenesis and osteogenesis.BMB Rep. 2015 Jun;48(6):319-23. doi: 10.5483/bmbrep.2015.48.6.206. BMB Rep. 2015. PMID: 25341923 Free PMC article. Review.
-
PPARγ and Wnt Signaling in Adipogenic and Osteogenic Differentiation of Mesenchymal Stem Cells.Curr Stem Cell Res Ther. 2016;11(3):216-25. doi: 10.2174/1574888x10666150519093429. Curr Stem Cell Res Ther. 2016. PMID: 25986621 Review.
Cited by
-
PPAR Gamma-Regulated MicroRNA 199a-5p Underlies Bone Marrow Adiposity in Aplastic Anemia.Mol Ther Nucleic Acids. 2019 Sep 6;17:678-687. doi: 10.1016/j.omtn.2019.07.005. Epub 2019 Jul 19. Mol Ther Nucleic Acids. 2019. PMID: 31400610 Free PMC article.
-
Bone marrow adiposity during pathologic bone loss: molecular mechanisms underlying the cellular events.J Mol Med (Berl). 2022 Feb;100(2):167-183. doi: 10.1007/s00109-021-02164-1. Epub 2021 Nov 9. J Mol Med (Berl). 2022. PMID: 34751809 Review.
-
The Potential Role of miRNAs as New Biomarkers for Osteoporosis.Int J Endocrinol. 2018 May 6;2018:2342860. doi: 10.1155/2018/2342860. eCollection 2018. Int J Endocrinol. 2018. PMID: 29853878 Free PMC article. Review.
-
MicroRNAs and Periodontal Homeostasis.J Dent Res. 2017 May;96(5):491-500. doi: 10.1177/0022034516685711. Epub 2017 Jan 9. J Dent Res. 2017. PMID: 28068481 Free PMC article. Review.
-
Altered function in cartilage derived mesenchymal stem cell leads to OA-related cartilage erosion.Am J Transl Res. 2016 Feb 15;8(2):433-46. eCollection 2016. Am J Transl Res. 2016. PMID: 27158337 Free PMC article.
References
-
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. - PubMed
-
- Tuli R, Tuli S, Nandi S, Wang ML, Alexander PG, Haleem-Smith H, et al. Characterization of multipotential mesenchymal progenitor cells derived from human trabecular bone. Stem Cells. 2003;21:681–693. - PubMed
-
- Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology. 2005;146:1226–1235. - PubMed
-
- Komori T. Regulation of bone development and maintenance by Runx2. Front Biosci. 2008;13:898–903. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

