Syntaxin 5-dependent retrograde transport to the trans-Golgi network is required for adeno-associated virus transduction
- PMID: 25410859
- PMCID: PMC4300741
- DOI: 10.1128/JVI.02520-14
Syntaxin 5-dependent retrograde transport to the trans-Golgi network is required for adeno-associated virus transduction
Abstract
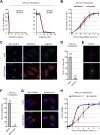
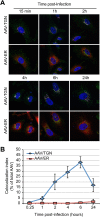
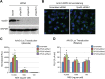
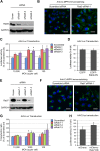
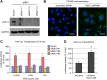
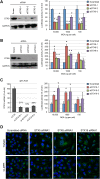
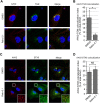
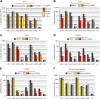
Intracellular transport of recombinant adeno-associated virus (AAV) is still incompletely understood. In particular, the trafficking steps preceding the release of incoming AAV particles from the endosomal system into the cytoplasm, allowing subsequent nuclear import and the initiation of gene expression, remain to be elucidated fully. Others and we previously showed that a significant proportion of viral particles are transported to the Golgi apparatus and that Golgi apparatus disruption caused by the drug brefeldin A efficiently blocks AAV serotype 2 (AAV2) transduction. However, because brefeldin A is known to exert pleiotropic effects on the entire endosomal system, the functional relevance of transport to the Golgi apparatus for AAV transduction remains to be established definitively. Here, we show that AAV2 trafficking toward the trans-Golgi network (TGN) and the Golgi apparatus correlates with transduction efficiency and relies on a nonclassical retrograde transport pathway that is independent of the retromer complex, late endosomes, and recycling endosomes. AAV2 transduction is unaffected by the knockdown of syntaxins 6 and 16, which are two major effectors in the retrograde transport of both exogenous and endogenous cargo. On the other hand, inhibition of syntaxin 5 function by small interfering RNA silencing or treatment with cyclized Retro-2 strongly decreases AAV2 transduction and transport to the Golgi apparatus. This inhibition of transduction is observed with several AAV serotypes and a number of primary and immortalized cells. Together, our data strongly suggest that syntaxin 5-mediated retrograde transport to the Golgi apparatus is a broadly conserved feature of AAV trafficking that appears to be independent of the identity of the receptors used for viral attachment.
Importance: Gene therapy constitutes a promising approach for the treatment of life-threatening conditions refractory to any other form of remedy. Adeno-associated virus (AAV) vectors are currently being evaluated for the treatment of diseases such as Duchenne muscular dystrophy, hemophilia, heart failure, Parkinson's disease, and others. Despite their promise as gene delivery vehicles, a better understanding of the biology of AAV-based vectors is necessary to improve further their efficacy. AAV vectors must reach the nucleus in order to deliver their genome, and their intracellular transport is not fully understood. Here, we dissect an important step of the intracellular journey of AAV by showing that retrograde transport of capsids to the trans-Golgi network is necessary for gene delivery. We show that the AAV trafficking route differs from that of known Golgi apparatus-targeted cargos, and we raise the possibility that this nonclassical pathway is shared by most AAV variants, regardless of their attachment receptors.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Host determinants of adeno-associated viral vector entry.Curr Opin Virol. 2017 Jun;24:124-131. doi: 10.1016/j.coviro.2017.06.003. Epub 2017 Jun 30. Curr Opin Virol. 2017. PMID: 28672171 Free PMC article. Review.
-
The Golgi Calcium ATPase Pump Plays an Essential Role in Adeno-associated Virus Trafficking and Transduction.J Virol. 2020 Oct 14;94(21):e01604-20. doi: 10.1128/JVI.01604-20. Print 2020 Oct 14. J Virol. 2020. PMID: 32817219 Free PMC article.
-
The golgin GCC88 is required for efficient retrograde transport of cargo from the early endosomes to the trans-Golgi network.Mol Biol Cell. 2007 Dec;18(12):4979-91. doi: 10.1091/mbc.e07-06-0622. Epub 2007 Oct 3. Mol Biol Cell. 2007. PMID: 17914056 Free PMC article.
-
Retrograde Transport from Early Endosomes to the trans-Golgi Network Enables Membrane Wrapping and Egress of Vaccinia Virus Virions.J Virol. 2016 Sep 12;90(19):8891-905. doi: 10.1128/JVI.01114-16. Print 2016 Oct 1. J Virol. 2016. PMID: 27466413 Free PMC article.
-
Intracellular trafficking of adeno-associated viral vectors.Gene Ther. 2005 Jun;12(11):873-80. doi: 10.1038/sj.gt.3302527. Gene Ther. 2005. PMID: 15829993 Review.
Cited by
-
Chemical Modulation of Endocytic Sorting Augments Adeno-associated Viral Transduction.J Biol Chem. 2016 Jan 8;291(2):939-47. doi: 10.1074/jbc.M115.687657. Epub 2015 Nov 2. J Biol Chem. 2016. PMID: 26527686 Free PMC article.
-
Host determinants of adeno-associated viral vector entry.Curr Opin Virol. 2017 Jun;24:124-131. doi: 10.1016/j.coviro.2017.06.003. Epub 2017 Jun 30. Curr Opin Virol. 2017. PMID: 28672171 Free PMC article. Review.
-
Quantitative Methods to Study Endocytosis and Retrograde Transport of Cargo Proteins.Methods Mol Biol. 2021;2233:53-70. doi: 10.1007/978-1-0716-1044-2_4. Methods Mol Biol. 2021. PMID: 33222127
-
Integrating transcriptomics and proteomics to analyze the immune microenvironment of cytomegalovirus associated ulcerative colitis and identify relevant biomarkers.BioData Min. 2024 Aug 27;17(1):26. doi: 10.1186/s13040-024-00382-0. BioData Min. 2024. PMID: 39192288 Free PMC article.
-
Adeno-associated virus infection and its impact in human health: an overview.Virol J. 2022 Oct 31;19(1):173. doi: 10.1186/s12985-022-01900-4. Virol J. 2022. PMID: 36316711 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

