A Phase III study of radiation therapy (RT) and O⁶-benzylguanine + BCNU versus RT and BCNU alone and methylation status in newly diagnosed glioblastoma and gliosarcoma: Southwest Oncology Group (SWOG) study S0001
- PMID: 25407559
- PMCID: PMC4465052
- DOI: 10.1007/s10147-014-0769-0
A Phase III study of radiation therapy (RT) and O⁶-benzylguanine + BCNU versus RT and BCNU alone and methylation status in newly diagnosed glioblastoma and gliosarcoma: Southwest Oncology Group (SWOG) study S0001
Abstract
Aims: To determine the efficacy of methylguanine methyltransferase (MGMT) depletion + BCNU [1,3-bis(2-chloroethyl)-1- nitrosourea: carmustine] therapy and the impact of methylation status in adults with glioblastoma multiforme (GBM) and gliosarcoma.
Methods: Methylation analysis was performed on GBM patients with adequate tissue samples. Patients with newly diagnosed GBM or gliosarcoma were eligible for this Phase III open-label clinical trial. At registration, patients were randomized to Arm 1, which consisted of therapy with O(6)-benzylguanine (O(6)-BG) + BCNU 40 mg/m(2) (reduced dose) + radiation therapy (RT) (O6BG + BCNU arm), or Arm 2, which consisted of therapy with BCNU 200 mg/m(2) + RT (BCNU arm).
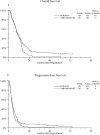
Results: A total of 183 patients with newly diagnosed GBM or gliosarcoma from 42 U.S. institutions were enrolled in this study. Of these, 90 eligible patients received O(6)-BG + BCNU + RT and 89 received BCNU + RT. The trial was halted at the first interim analysis in accordance with the guidelines for stopping the study due to futility (<40 % improvement among patients on the O6BG + BCNU arm). Following adjustment for stratification factors, there was no significant difference in overall survival (OS) or progression-free survival (PFS) between the two groups (one sided p = 0.94 and p = 0.88, respectively). Median OS was 11 [95 % confidence interval (CI) 8-13] months for patients in the O6BG + BCNU arm and 10 (95 % CI 8-12) months for those in the BCNU arm. PFS was 4 months for patients in each arm. Adverse events were reported in both arms, with significantly more grade 4 and 5 events in the experimental arm.
Conclusions: The addition of O(6)-BG to the standard regimen of radiation and BCNU for the treatment patients with newly diagnosed GBM and gliosarcoma did not provide added benefit and in fact caused additional toxicity.
Trial registration: ClinicalTrials.gov NCT00017147.
Similar articles
-
Phase II and pharmacogenomics study of enzastaurin plus temozolomide during and following radiation therapy in patients with newly diagnosed glioblastoma multiforme and gliosarcoma.Neuro Oncol. 2011 Dec;13(12):1331-8. doi: 10.1093/neuonc/nor130. Epub 2011 Sep 6. Neuro Oncol. 2011. PMID: 21896554 Free PMC article. Clinical Trial.
-
Phase III trial of carmustine and cisplatin compared with carmustine alone and standard radiation therapy or accelerated radiation therapy in patients with glioblastoma multiforme: North Central Cancer Treatment Group 93-72-52 and Southwest Oncology Group 9503 Trials.J Clin Oncol. 2006 Aug 20;24(24):3871-9. doi: 10.1200/JCO.2005.04.6979. J Clin Oncol. 2006. PMID: 16921039 Clinical Trial.
-
MGMT promoter methylation status and prognosis of patients with primary or recurrent glioblastoma treated with carmustine wafers.Br J Neurosurg. 2013 Dec;27(6):772-8. doi: 10.3109/02688697.2013.791664. Epub 2013 May 11. Br J Neurosurg. 2013. PMID: 23662801 Clinical Trial.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.Health Technol Assess. 2007 Nov;11(45):iii-iv, ix-221. doi: 10.3310/hta11450. Health Technol Assess. 2007. PMID: 17999840 Review.
-
Gliadel wafer implantation combined with standard radiotherapy and concurrent followed by adjuvant temozolomide for treatment of newly diagnosed high-grade glioma: a systematic literature review.World J Surg Oncol. 2016 Aug 24;14(1):225. doi: 10.1186/s12957-016-0975-5. World J Surg Oncol. 2016. PMID: 27557526 Free PMC article. Review.
Cited by
-
Glioblastoma Therapy: Past, Present and Future.Int J Mol Sci. 2024 Feb 21;25(5):2529. doi: 10.3390/ijms25052529. Int J Mol Sci. 2024. PMID: 38473776 Free PMC article. Review.
-
Optimized Image-Based Surrogate Endpoints in Targeted Therapies for Glioblastoma: A Systematic Review and Meta-Analysis of Phase III Randomized Controlled Trials.Korean J Radiol. 2020 Apr;21(4):471-482. doi: 10.3348/kjr.2019.0839. Korean J Radiol. 2020. PMID: 32193895 Free PMC article.
-
Treatment of newly diagnosed glioblastoma in the elderly: a network meta-analysis.Cochrane Database Syst Rev. 2020 Mar 23;3(3):CD013261. doi: 10.1002/14651858.CD013261.pub2. Cochrane Database Syst Rev. 2020. PMID: 32202316 Free PMC article.
-
DNA damage response inhibitors in cancer therapy: lessons from the past, current status and future implications.Nat Rev Drug Discov. 2025 Jan;24(1):19-39. doi: 10.1038/s41573-024-01060-w. Epub 2024 Nov 12. Nat Rev Drug Discov. 2025. PMID: 39533099 Review.
-
MGMT inhibition regulates radioresponse in GBM, GSC, and melanoma.Sci Rep. 2024 May 29;14(1):12363. doi: 10.1038/s41598-024-61240-x. Sci Rep. 2024. PMID: 38811596 Free PMC article.
References
-
- Walker MD, Alexander E, Jr, Hunt WE, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49:333–343. - PubMed
-
- Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. International journal of radiation oncology, biology, physics. 1979;5:1725–1731. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- N01 CA004919/CA/NCI NIH HHS/United States
- CA22433/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- CA35281/CA/NCI NIH HHS/United States
- N01 CA045807/CA/NCI NIH HHS/United States
- CA45808/CA/NCI NIH HHS/United States
- CA35262/CA/NCI NIH HHS/United States
- U10 CA046368/CA/NCI NIH HHS/United States
- CA37981/CA/NCI NIH HHS/United States
- U10 CA022433/CA/NCI NIH HHS/United States
- N01 CA035176/CA/NCI NIH HHS/United States
- CA76462/CA/NCI NIH HHS/United States
- U10 CA012644/CA/NCI NIH HHS/United States
- CA35128/CA/NCI NIH HHS/United States
- CA35261/CA/NCI NIH HHS/United States
- U10 CA037981/CA/NCI NIH HHS/United States
- U10 CA004919/CA/NCI NIH HHS/United States
- N01 CA035431/CA/NCI NIH HHS/United States
- U10 CA046113/CA/NCI NIH HHS/United States
- U10 CA045560/CA/NCI NIH HHS/United States
- CA12644/CA/NCI NIH HHS/United States
- U10 CA035128/CA/NCI NIH HHS/United States
- CA20319/CA/NCI NIH HHS/United States
- N01 CA013612/CA/NCI NIH HHS/United States
- U10 CA035192/CA/NCI NIH HHS/United States
- U10 CA045808/CA/NCI NIH HHS/United States
- U10 CA013612/CA/NCI NIH HHS/United States
- CA45377/CA/NCI NIH HHS/United States
- U10 CA074647/CA/NCI NIH HHS/United States
- U10 CA035281/CA/NCI NIH HHS/United States
- CA58861/CA/NCI NIH HHS/United States
- CA35090/CA/NCI NIH HHS/United States
- CA46282/CA/NCI NIH HHS/United States
- U10 CA035261/CA/NCI NIH HHS/United States
- U10 CA035178/CA/NCI NIH HHS/United States
- U10 CA045450/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- U10 CA046282/CA/NCI NIH HHS/United States
- CA45450/CA/NCI NIH HHS/United States
- U10 CA035262/CA/NCI NIH HHS/United States
- CA46368/CA/NCI NIH HHS/United States
- N01 CA035178/CA/NCI NIH HHS/United States
- N01 CA038926/CA/NCI NIH HHS/United States
- U10 CA067575/CA/NCI NIH HHS/United States
- CA74811/CA/NCI NIH HHS/United States
- U10 CA045377/CA/NCI NIH HHS/United States
- CA35192/CA/NCI NIH HHS/United States
- CA74647/CA/NCI NIH HHS/United States
- U10 CA020319/CA/NCI NIH HHS/United States
- CA46113/CA/NCI NIH HHS/United States
- U10 CA038926/CA/NCI NIH HHS/United States
- U10 CA042777/CA/NCI NIH HHS/United States
- U10 CA074811/CA/NCI NIH HHS/United States
- U10 CA035431/CA/NCI NIH HHS/United States
- U10 CA095860/CA/NCI NIH HHS/United States
- CA42777/CA/NCI NIH HHS/United States
- CA52654/CA/NCI NIH HHS/United States
- N01 CA067575/CA/NCI NIH HHS/United States
- U10 CA052654/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- U10 CA180819/CA/NCI NIH HHS/United States
- U10 CA035176/CA/NCI NIH HHS/United States
- U10 CA035090/CA/NCI NIH HHS/United States
- CA95860/CA/NCI NIH HHS/United States
- U10 CA058861/CA/NCI NIH HHS/United States
- U10 CA045807/CA/NCI NIH HHS/United States
- N01 CA045560/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous