MUNC, a long noncoding RNA that facilitates the function of MyoD in skeletal myogenesis
- PMID: 25403490
- PMCID: PMC4285423
- DOI: 10.1128/MCB.01079-14
MUNC, a long noncoding RNA that facilitates the function of MyoD in skeletal myogenesis
Abstract
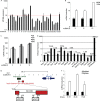
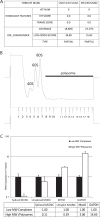
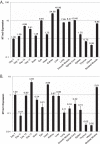
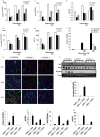
An in silico screen for myogenic long noncoding RNAs (lncRNAs) revealed nine lncRNAs that are upregulated more than 10-fold in myotubes versus levels in myoblasts. One of these lncRNAs, MyoD upstream noncoding (MUNC, also known as DRR(eRNA)), is encoded 5 kb upstream of the transcription start site of MyoD, a myogenic transcription factor gene. MUNC is specifically expressed in skeletal muscle and exists as in unspliced and spliced isoforms, and its 5' end overlaps with the cis-acting distal regulatory region (DRR) of MyoD. Small interfering RNA (siRNA) of MUNC reduced myoblast differentiation and specifically reduced the association of MyoD to the DRR enhancer and myogenin promoter but not to another MyoD-dependent enhancer. Stable overexpression of MUNC from a heterologous promoter increased endogenous MyoD, Myogenin, and Myh3 (myosin heavy chain, [MHC] gene) mRNAs but not the cognate proteins, suggesting that MUNC can act in trans to promote gene expression but that this activity does not require an induction of MyoD protein. MUNC also stimulates the transcription of other genes that are not recognized as MyoD-inducible genes. Knockdown of MUNC in vivo impaired murine muscle regeneration, implicating MUNC in primary satellite cell differentiation in the animal. We also discovered a human MUNC that is induced during differentiation of myoblasts and whose knockdown decreases differentiation, suggesting an evolutionarily conserved role of MUNC lncRNA in myogenesis. Although MUNC overlaps with the DRR enhancer, our results suggest that MUNC is not a classic cis-acting enhancer RNA (e-RNA) acting exclusively by stimulating the neighboring MyoD gene but more like a promyogenic lncRNA that acts directly or indirectly on multiple promoters to increase myogenic gene expression.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures





Similar articles
-
MUNC, an Enhancer RNA Upstream from the MYOD Gene, Induces a Subgroup of Myogenic Transcripts in trans Independently of MyoD.Mol Cell Biol. 2018 Sep 28;38(20):e00655-17. doi: 10.1128/MCB.00655-17. Print 2018 Oct 15. Mol Cell Biol. 2018. PMID: 30037979 Free PMC article.
-
S100B protein in myoblasts modulates myogenic differentiation via NF-kappaB-dependent inhibition of MyoD expression.J Cell Physiol. 2010 Apr;223(1):270-82. doi: 10.1002/jcp.22035. J Cell Physiol. 2010. PMID: 20069545
-
Tip60 regulates myoblast differentiation by enhancing the transcriptional activity of MyoD via their physical interactions.FEBS J. 2011 Nov;278(22):4394-404. doi: 10.1111/j.1742-4658.2011.08362.x. Epub 2011 Oct 17. FEBS J. 2011. PMID: 21936881
-
[Interactions of proliferation and differentiation signaling pathways in myogenesis].Postepy Hig Med Dosw (Online). 2014 May 8;68:516-26. doi: 10.5604/17322693.1101617. Postepy Hig Med Dosw (Online). 2014. PMID: 24864103 Review. Polish.
-
Regulation of Skeletal Myoblast Differentiation by Drebrin.Adv Exp Med Biol. 2017;1006:361-373. doi: 10.1007/978-4-431-56550-5_22. Adv Exp Med Biol. 2017. PMID: 28865032 Review.
Cited by
-
Clinical value of non-coding RNAs in cardiovascular, pulmonary, and muscle diseases.Am J Physiol Cell Physiol. 2020 Jan 1;318(1):C1-C28. doi: 10.1152/ajpcell.00078.2019. Epub 2019 Sep 4. Am J Physiol Cell Physiol. 2020. PMID: 31483703 Free PMC article. Review.
-
RNA-sequencing analysis reveals the potential contribution of lncRNAs in palmitic acid-induced insulin resistance of skeletal muscle cells.Biosci Rep. 2020 Jan 31;40(1):BSR20192523. doi: 10.1042/BSR20192523. Biosci Rep. 2020. PMID: 31833538 Free PMC article.
-
LncRNA Functions as a New Emerging Epigenetic Factor in Determining the Fate of Stem Cells.Front Genet. 2020 Mar 31;11:277. doi: 10.3389/fgene.2020.00277. eCollection 2020. Front Genet. 2020. PMID: 32296461 Free PMC article. Review.
-
Long noncoding RNAs and their roles in skeletal muscle fate determination.Noncoding RNA Investig. 2017 Dec;1:24. doi: 10.21037/ncri.2017.12.01. Epub 2017 Dec 13. Noncoding RNA Investig. 2017. PMID: 29451560 Free PMC article.
-
RNA sequencing reveals potential interacting networks between the altered transcriptome and ncRNome in the skeletal muscle of diabetic mice.Biosci Rep. 2021 Jul 30;41(7):BSR20210495. doi: 10.1042/BSR20210495. Biosci Rep. 2021. PMID: 34190986 Free PMC article.
References
-
- Clark MB, Amaral PP, Schlesinger FJ, Dinger ME, Taft RJ, Rinn JL, Ponting CP, Stadler PF, Morris KV, Morillon A, Rozowsky JS, Gerstein MB, Wahlestedt C, Hayashizaki Y, Carninci P, Gingeras TR, Mattick JS. 2011. The reality of pervasive transcription. PLoS Biol 9:e1000625. doi:10.1371/journal.pbio.1000625. - DOI - PMC - PubMed
-
- Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG, Lagarde J, Veeravalli L, Ruan X, Ruan Y, Lassmann T, Carninci P, Brown JB, Lipovich L, Gonzalez JM, Thomas M, Davis CA, Shiekhattar R, Gingeras TR, Hubbard TJ, Notredame C, Harrow J, Guigó R. 2012. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res 22:1775–1789. doi:10.1101/gr.132159.111. - DOI - PMC - PubMed
-
- Guttman M, Garber M, Levin JZ, Donaghey J, Robinson J, Adiconis X, Fan L, Koziol MJ, Gnirke A, Nusbaum C, Rinn JL, Lander ES, Regev A. 2010. Ab initio reconstruction of cell type-specific transcriptomes in mouse reveals the conserved multi-exonic structure of lincRNAs. Nat Biotechnol 28:503–510. doi:10.1038/nbt.1633. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
