Viral infection. Prevention and cure of rotavirus infection via TLR5/NLRC4-mediated production of IL-22 and IL-18
- PMID: 25395539
- PMCID: PMC4788408
- DOI: 10.1126/science.1256999
Viral infection. Prevention and cure of rotavirus infection via TLR5/NLRC4-mediated production of IL-22 and IL-18
Abstract
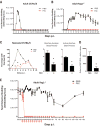
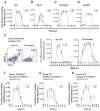
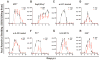
Activators of innate immunity may have the potential to combat a broad range of infectious agents. We report that treatment with bacterial flagellin prevented rotavirus (RV) infection in mice and cured chronically RV-infected mice. Protection was independent of adaptive immunity and interferon (IFN, type I and II) and required flagellin receptors Toll-like receptor 5 (TLR5) and NOD-like receptor C4 (NLRC4). Flagellin-induced activation of TLR5 on dendritic cells elicited production of the cytokine interleukin-22 (IL-22), which induced a protective gene expression program in intestinal epithelial cells. Flagellin also induced NLRC4-dependent production of IL-18 and immediate elimination of RV-infected cells. Administration of IL-22 and IL-18 to mice fully recapitulated the capacity of flagellin to prevent or eliminate RV infection and thus holds promise as a broad-spectrum antiviral agent.
Copyright © 2014, American Association for the Advancement of Science.
Figures

Similar articles
-
TLR5 or NLRC4 is necessary and sufficient for promotion of humoral immunity by flagellin.Eur J Immunol. 2010 Dec;40(12):3528-34. doi: 10.1002/eji.201040421. Eur J Immunol. 2010. PMID: 21072873 Free PMC article.
-
IL-22-induced cell extrusion and IL-18-induced cell death prevent and cure rotavirus infection.Sci Immunol. 2020 Oct 2;5(52):eabd2876. doi: 10.1126/sciimmunol.abd2876. Sci Immunol. 2020. PMID: 33008915 Free PMC article.
-
Flagellin treatment protects against chemicals, bacteria, viruses, and radiation.J Immunol. 2008 Jun 15;180(12):8280-5. doi: 10.4049/jimmunol.180.12.8280. J Immunol. 2008. PMID: 18523294
-
Redundant and cooperative interactions between TLR5 and NLRC4 in protective lung mucosal immunity against Pseudomonas aeruginosa.J Innate Immun. 2015;7(2):177-86. doi: 10.1159/000367790. Epub 2014 Nov 12. J Innate Immun. 2015. PMID: 25402425 Free PMC article.
-
Compartmentalized Antimicrobial Defenses in Response to Flagellin.Trends Microbiol. 2018 May;26(5):423-435. doi: 10.1016/j.tim.2017.10.008. Epub 2017 Nov 22. Trends Microbiol. 2018. PMID: 29173868 Review.
Cited by
-
A Double Edged Sword Role of Interleukin-22 in Wound Healing and Tissue Regeneration.Front Immunol. 2020 Sep 17;11:2148. doi: 10.3389/fimmu.2020.02148. eCollection 2020. Front Immunol. 2020. PMID: 33042126 Free PMC article. Review.
-
Roseburia intestinalis‑derived flagellin ameliorates colitis by targeting miR‑223‑3p‑mediated activation of NLRP3 inflammasome and pyroptosis.Mol Med Rep. 2020 Oct;22(4):2695-2704. doi: 10.3892/mmr.2020.11351. Epub 2020 Jul 23. Mol Med Rep. 2020. PMID: 32700754 Free PMC article.
-
Segmented filamentous bacteria impede rotavirus infection via retinoic acid receptor-mediated signaling.Gut Microbes. 2023 Jan-Dec;15(1):2174407. doi: 10.1080/19490976.2023.2174407. Gut Microbes. 2023. PMID: 36740862 Free PMC article.
-
Diversity and dynamism of IgA-microbiota interactions.Nat Rev Immunol. 2021 Aug;21(8):514-525. doi: 10.1038/s41577-021-00506-1. Epub 2021 Feb 10. Nat Rev Immunol. 2021. PMID: 33568782 Review.
-
The Complex Interactions Between Rotavirus and the Gut Microbiota.Front Cell Infect Microbiol. 2021 Jan 8;10:586751. doi: 10.3389/fcimb.2020.586751. eCollection 2020. Front Cell Infect Microbiol. 2021. PMID: 33489932 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- AI107943/AI/NIAID NIH HHS/United States
- R01 DK061417/DK/NIDDK NIH HHS/United States
- DK061417/DK/NIDDK NIH HHS/United States
- R56 AI080656/AI/NIAID NIH HHS/United States
- R01 AI080656/AI/NIAID NIH HHS/United States
- R37 AI038296/AI/NIAID NIH HHS/United States
- AI038296/AI/NIAID NIH HHS/United States
- DK064730/DK/NIDDK NIH HHS/United States
- R21 AI107943/AI/NIAID NIH HHS/United States
- R01 DK064730/DK/NIDDK NIH HHS/United States
- R01 AI038296/AI/NIAID NIH HHS/United States
- P30 DK056338/DK/NIDDK NIH HHS/United States
- AI080656/AI/NIAID NIH HHS/United States
- DK56338/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

