Loss of microRNA-106b-25 cluster promotes atrial fibrillation by enhancing ryanodine receptor type-2 expression and calcium release
- PMID: 25389315
- PMCID: PMC4270890
- DOI: 10.1161/CIRCEP.114.001973
Loss of microRNA-106b-25 cluster promotes atrial fibrillation by enhancing ryanodine receptor type-2 expression and calcium release
Abstract
Background: Enhanced sarcoplasmic reticulum Ca(2+)-leak via ryanodine receptor type-2 (RyR2) contributes to the pathogenesis of atrial fibrillation (AF). Recent studies have shown that the level of RyR2 protein is elevated in atria of patients with paroxysmal AF, suggesting that microRNA-mediated post-transcriptional regulation of RyR2 might be an underlying mechanism. Bioinformatic analysis suggests that miR-106b and miR-93, members of the miR-106b-25 cluster, could bind to RyR2-3'-untranslated region and suppress its translation. Thus, we tested the hypothesis that loss of the miR-106b-25 cluster promotes AF via enhanced RyR2-mediated sarcoplasmic reticulum Ca(2+)-leak.
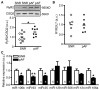
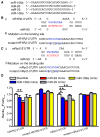
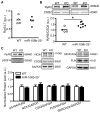
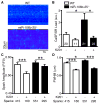
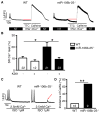
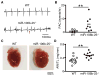
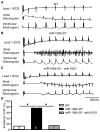
Methods and results: Quantitative real-time polymerase chain reaction showed that the levels of mature miR-106b, miR-93, and miR-25 were lower in atria of patients with paroxysmal AF when compared with patients in sinus rhythm. In vitro assay showed that miR-93 reduced RyR2-3'-untranslated region luciferase activity. Total RyR2 protein in atrial tissue of miR-106b-25(-/-) mice was increased by 42% when compared with wild-type littermates but still maintained a normal subcellular distribution. Ca(2+)-spark frequency and total sarcoplasmic reticulum Ca(2+)-leak were increased in atrial myocytes of miR-106b-25(-/-) mice. Telemetry ECG recordings revealed that miR-106b-25(-/-) mice exhibited more frequent atrial ectopy and were also more susceptible to pacing-induced AF than wild-type littermates. Increased sarcoplasmic reticulum Ca(2+)-release and AF susceptibility in miR-106b-25(-/-) mice were abolished by the RyR2 blocker K201.
Conclusions: These results suggest that miR-106b-25 cluster-mediated post-transcriptional regulation of RyR2 is a potential molecular mechanism involved in paroxysmal AF pathogenesis. As such, the miR-106b-25 cluster could be a novel gene-therapy target in AF associated with enhanced RyR2 expression.
Keywords: atrial fibrillation; microRNA; ryanodine receptor calcium release channel.
© 2014 American Heart Association, Inc.
Conflict of interest statement
Figures
Similar articles
-
Loss of SPEG Inhibitory Phosphorylation of Ryanodine Receptor Type-2 Promotes Atrial Fibrillation.Circulation. 2020 Sep 22;142(12):1159-1172. doi: 10.1161/CIRCULATIONAHA.120.045791. Epub 2020 Jul 20. Circulation. 2020. PMID: 32683896 Free PMC article.
-
Loss of Protein Phosphatase 1 Regulatory Subunit PPP1R3A Promotes Atrial Fibrillation.Circulation. 2019 Aug 20;140(8):681-693. doi: 10.1161/CIRCULATIONAHA.119.039642. Epub 2019 Jun 12. Circulation. 2019. PMID: 31185731 Free PMC article.
-
Increased expression of ryanodine receptor type-2 during atrial fibrillation by miR-106-25 cluster independent mechanism.Exp Cell Res. 2019 Feb 15;375(2):113-117. doi: 10.1016/j.yexcr.2018.11.025. Epub 2018 Nov 27. Exp Cell Res. 2019. PMID: 30496756
-
Calcium-mediated cellular triggered activity in atrial fibrillation.J Physiol. 2017 Jun 15;595(12):4001-4008. doi: 10.1113/JP273048. Epub 2017 Mar 22. J Physiol. 2017. PMID: 28181690 Free PMC article. Review.
-
Calcium signalling silencing in atrial fibrillation.J Physiol. 2017 Jun 15;595(12):4009-4017. doi: 10.1113/JP273045. Epub 2017 May 14. J Physiol. 2017. PMID: 28332202 Free PMC article. Review.
Cited by
-
Electrical PR Interval Variation Predicts New Occurrence of Atrial Fibrillation in Patients With Frequent Premature Atrial Contractions.Medicine (Baltimore). 2016 Apr;95(14):e3249. doi: 10.1097/MD.0000000000003249. Medicine (Baltimore). 2016. PMID: 27057868 Free PMC article.
-
Biomarkers in the clinical management of patients with atrial fibrillation and heart failure.J Geriatr Cardiol. 2021 Nov 28;18(11):908-951. doi: 10.11909/j.issn.1671-5411.2021.11.010. J Geriatr Cardiol. 2021. PMID: 34908928 Free PMC article.
-
MEF2C Directly Interacts with Pre-miRNAs and Distinct RNPs to Post-Transcriptionally Regulate miR-23a-miR-27a-miR-24-2 microRNA Cluster Member Expression.Noncoding RNA. 2024 May 17;10(3):32. doi: 10.3390/ncrna10030032. Noncoding RNA. 2024. PMID: 38804364 Free PMC article.
-
MicroRNA-20b suppresses the expression of ZFP-148 in viral myocarditis.Mol Cell Biochem. 2017 May;429(1-2):199-210. doi: 10.1007/s11010-017-2947-7. Epub 2017 Feb 28. Mol Cell Biochem. 2017. Retraction in: Mol Cell Biochem. 2023 Jun;478(6):1413. doi: 10.1007/s11010-022-04598-8. PMID: 28247213 Retracted.
-
Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation.Circulation. 2018 Nov 13;138(20):2227-2242. doi: 10.1161/CIRCULATIONAHA.118.035202. Circulation. 2018. PMID: 29802206 Free PMC article.
References
-
- Nattel S. Experimental evidence for proarrhythmic mechanisms of antiarrhythmic drugs. Cardiovasc Res. 1998;37:567–577. - PubMed
-
- Li N, Chiang DY, Wang S, Wang Q, Sun L, Voigt N, Respress JL, Ather S, Skapura DG, Jordan VK, Horrigan FT, Schmitz W, Muller FU, Valderrabano M, Nattel S, Dobrev D, Wehrens XH. Ryanodine receptor-mediated calcium leak drives progressive development of an atrial fibrillation substrate in a transgenic mouse model. Circulation. 2014;129:1276–1285. - PMC - PubMed
-
- Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Muller FU, Schmitz W, Schotten U, Anderson ME, Valderrabano M, Dobrev D, Wehrens XH. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL091947/HL/NHLBI NIH HHS/United States
- K12 GM084897/GM/NIGMS NIH HHS/United States
- R01 HL118761/HL/NHLBI NIH HHS/United States
- HL089598/HL/NHLBI NIH HHS/United States
- R42 HL114206/HL/NHLBI NIH HHS/United States
- R25 GM056929/GM/NIGMS NIH HHS/United States
- R01 HL089598/HL/NHLBI NIH HHS/United States
- R01 DE023177/DE/NIDCR NIH HHS/United States
- HL117641/HL/NHLBI NIH HHS/United States
- R01 HL117641/HL/NHLBI NIH HHS/United States
- R01 CA190467/CA/NCI NIH HHS/United States
- R01 HL091947/HL/NHLBI NIH HHS/United States
- R25 GM069234/GM/NIGMS NIH HHS/United States
- HL118761/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

