Endothelial cell antibodies associated with novel targets and increased rejection
- PMID: 25381426
- PMCID: PMC4413753
- DOI: 10.1681/ASN.2013121277
Endothelial cell antibodies associated with novel targets and increased rejection
Abstract
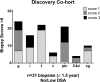
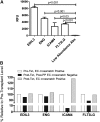
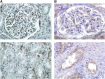
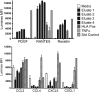
The initial contact point between a recipient's immune system and a transplanted graft is the vascular endothelium. Clinical studies suggest a pathogenic role for non-HLA antiendothelial cell antibodies (AECAs) in allograft rejection; however, evidence linking AECAs of known specificity to in vivo vascular injury is lacking. Here, we used high-density protein arrays to identify target antigens for AECAs isolated from the sera of recipients of kidney transplants experiencing antibody-mediated rejection in the absence of donor-specific HLA antibodies. Four antigenic targets expressed on endothelial cells were identified: endoglin, Fms-like tyrosine kinase-3 ligand, EGF-like repeats and discoidin I-like domains 3, and intercellular adhesion molecule 4; the first three have been implicated in endothelial cell activation and leukocyte extravasation. To validate these findings, ELISAs were constructed, and sera from an additional 150 renal recipients were tested. All four AECAs were detected in 24% of pretransplant sera, and they were associated with post-transplant donor-specific HLA antibodies, antibody-mediated rejection, and early transplant glomerulopathy. AECA stimulation of endothelial cell cultures increased adhesion molecule expression and production of inflammatory cytokines: regulated on activation, normal T cell expressed and secreted PDGF and RESISTIN. These correlations between in vitro experiments and in vivo histopathology suggest that AECAs activate the vascular endothelium, amplifying the alloimmune response and increasing microvascular damage. Given the growing number of transplant candidates, a better understanding of the antigenic targets, beyond HLA, and mechanisms of immune injury will be essential for improving long-term allograft survival.
Keywords: acute allograft rejection; endothelial cells; immunology and pathology; kidney transplantation.
Copyright © 2015 by the American Society of Nephrology.
Figures

Comment in
-
Renal allograft rejection: pieces of the puzzle.J Am Soc Nephrol. 2015 May;26(5):1004-5. doi: 10.1681/ASN.2014090932. Epub 2014 Nov 7. J Am Soc Nephrol. 2015. PMID: 25381428 Free PMC article. No abstract available.
Similar articles
-
Analysis of Sera of Recipients with Allograft Rejection Indicates That Keratin 1 Is the Target of Anti-Endothelial Antibodies.J Immunol Res. 2017;2017:8679841. doi: 10.1155/2017/8679841. Epub 2017 Feb 7. J Immunol Res. 2017. PMID: 28265584 Free PMC article.
-
Isolated De Novo Antiendothelial Cell Antibodies and Kidney Transplant Rejection.Am J Kidney Dis. 2016 Dec;68(6):933-943. doi: 10.1053/j.ajkd.2016.07.019. Epub 2016 Sep 3. Am J Kidney Dis. 2016. PMID: 27599627
-
An association between antibodies specific for endothelial cells and renal transplant failure.Transpl Immunol. 1998 Jun;6(2):101-6. doi: 10.1016/s0966-3274(98)80024-5. Transpl Immunol. 1998. PMID: 9777698
-
Non-HLA antibodies against endothelial targets bridging allo- and autoimmunity.Kidney Int. 2016 Aug;90(2):280-288. doi: 10.1016/j.kint.2016.03.019. Epub 2016 May 14. Kidney Int. 2016. PMID: 27188505 Review.
-
Pathogenesis of non-HLA antibodies in solid organ transplantation: Where do we stand?Hum Immunol. 2016 Nov;77(11):1055-1062. doi: 10.1016/j.humimm.2016.05.021. Epub 2016 May 26. Hum Immunol. 2016. PMID: 27237040 Review.
Cited by
-
Impact of Non-Human Leukocyte Antigen-Specific Antibodies in Kidney and Heart Transplantation.Front Immunol. 2017 Apr 13;8:434. doi: 10.3389/fimmu.2017.00434. eCollection 2017. Front Immunol. 2017. PMID: 28450866 Free PMC article. Review.
-
Novel Non-Histocompatibility Antigen Mismatched Variants Improve the Ability to Predict Antibody-Mediated Rejection Risk in Kidney Transplant.Front Immunol. 2017 Dec 5;8:1687. doi: 10.3389/fimmu.2017.01687. eCollection 2017. Front Immunol. 2017. PMID: 29259604 Free PMC article.
-
Chronic transplant glomerulopathy: New insights into pathogenesis.Clin Transplant. 2021 Mar;35(3):e14214. doi: 10.1111/ctr.14214. Epub 2021 Feb 6. Clin Transplant. 2021. PMID: 33389755 Free PMC article. Review.
-
Antibody Subclass Repertoire and Graft Outcome Following Solid Organ Transplantation.Front Immunol. 2016 Oct 24;7:433. doi: 10.3389/fimmu.2016.00433. eCollection 2016. Front Immunol. 2016. PMID: 27822209 Free PMC article. Review.
-
Endothelin Type A Receptor Antibodies Are Associated With Angiotensin II Type 1 Receptor Antibodies, Vascular Inflammation, and Decline in Renal Function in Pediatric Kidney Transplantation.Kidney Int Rep. 2020 Sep 6;5(11):1925-1936. doi: 10.1016/j.ekir.2020.09.004. eCollection 2020 Nov. Kidney Int Rep. 2020. PMID: 33163713 Free PMC article.
References
-
- McKenna RM, Takemoto SK, Terasaki PI: Anti-HLA antibodies after solid organ transplantation. Transplantation 69: 319–326, 2000 - PubMed
-
- Mengel M, Sis B, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Cendales L, Demetris AJ, Drachenberg CB, Farver CF, Rodriguez ER, Wallace WD, Glotz D, Banff meeting report writing committee : Banff 2011 Meeting report: New concepts in antibody-mediated rejection. Am J Transplant 12: 563–570, 2012 - PMC - PubMed
-
- Sis B, Jhangri GS, Riopel J, Chang J, de Freitas DG, Hidalgo L, Mengel M, Matas A, Halloran PF: A new diagnostic algorithm for antibody-mediated microcirculation inflammation in kidney transplants. Am J Transplant 12: 1168–1179, 2012 - PubMed
-
- Loupy A, Hill GS, Jordan SC: The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol 8: 348–357, 2012 - PubMed
-
- Morrell CN, Murata K, Swaim AM, Mason E, Martin TV, Thompson LE, Ballard M, Fox-Talbot K, Wasowska B, Baldwin WM, 3rd: In vivo platelet-endothelial cell interactions in response to major histocompatibility complex alloantibody. Circ Res 102: 777–785, 2008 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

