Mitochondrial MKP1 is a target for therapy-resistant HER2-positive breast cancer cells
- PMID: 25377473
- PMCID: PMC4267894
- DOI: 10.1158/0008-5472.CAN-14-0844
Mitochondrial MKP1 is a target for therapy-resistant HER2-positive breast cancer cells
Abstract
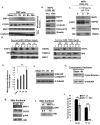
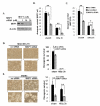
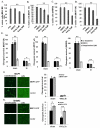
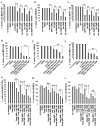
The MAPK phosphatase MKP1 (DUSP1) is overexpressed in many human cancers, including chemoresistant and radioresistant breast cancer cells, but its functional contributions in these settings are unclear. Here, we report that after cell irradiation, MKP1 translocates into mitochondria, where it prevents apoptotic induction by limiting accumulation of phosphorylated active forms of the stress kinase JNK. Increased levels of mitochondrial MKP1 after irradiation occurred in the mitochondrial inner membrane space. Notably, cell survival regulated by mitochondrial MKP1 was responsible for conferring radioresistance in HER2-overexpressing breast cancer cells, due to the fact that MKP1 serves as a major downstream effector in the HER2-activated RAF-MEK-ERK pathway. Clinically, we documented MKP1 expression exclusively in HER2-positive breast tumors, relative to normal adjacent tissue from the same patients. MKP1 overexpression was also detected in irradiated HER2-positive breast cancer stem-like cells (HER2(+)/CD44(+)/CD24(-/low)) isolated from a radioresistant breast cancer cell population after long-term radiation treatment. MKP1 silencing reduced clonogenic survival and enhanced radiosensitivity in these stem-like cells. Combined inhibition of MKP1 and HER2 enhanced cell killing in breast cancer. Together, our findings identify a new mechanism of resistance in breast tumors and reveal MKP1 as a novel therapeutic target for radiosensitization.
©2014 American Association for Cancer Research.
Figures


Similar articles
-
HER2-associated radioresistance of breast cancer stem cells isolated from HER2-negative breast cancer cells.Clin Cancer Res. 2012 Dec 15;18(24):6634-47. doi: 10.1158/1078-0432.CCR-12-1436. Epub 2012 Oct 22. Clin Cancer Res. 2012. PMID: 23091114 Free PMC article.
-
Hyperglycaemia Stress-Induced Renal Injury is Caused by Extensive Mitochondrial Fragmentation, Attenuated MKP1 Signalling, and Activated JNK-CaMKII-Fis1 Biological Axis.Cell Physiol Biochem. 2018;51(4):1778-1798. doi: 10.1159/000495681. Epub 2018 Nov 30. Cell Physiol Biochem. 2018. PMID: 30504726
-
Regulation of cell survival by HUNK mediates breast cancer resistance to HER2 inhibitors.Breast Cancer Res Treat. 2015 Jan;149(1):91-8. doi: 10.1007/s10549-014-3227-9. Epub 2014 Dec 17. Breast Cancer Res Treat. 2015. PMID: 25515931 Free PMC article.
-
Role of DUSP1/MKP1 in tumorigenesis, tumor progression and therapy.Cancer Med. 2016 Aug;5(8):2061-8. doi: 10.1002/cam4.772. Epub 2016 May 26. Cancer Med. 2016. PMID: 27227569 Free PMC article. Review.
-
Biologicals to direct nanotherapeutics towards HER2-positive breast cancers.Nanomedicine. 2020 Jul;27:102197. doi: 10.1016/j.nano.2020.102197. Epub 2020 Apr 7. Nanomedicine. 2020. PMID: 32275958 Review.
Cited by
-
Fatty acid oxidation fuels glioblastoma radioresistance with CD47-mediated immune evasion.Nat Commun. 2022 Mar 21;13(1):1511. doi: 10.1038/s41467-022-29137-3. Nat Commun. 2022. PMID: 35314680 Free PMC article.
-
Aldo-keto reductase family 1 member C3 mediates radioresistance of esophageal cancer cells through suppressing MAPK and AKT signaling.BMC Cancer. 2024 Oct 7;24(1):1236. doi: 10.1186/s12885-024-13012-z. BMC Cancer. 2024. PMID: 39375680 Free PMC article.
-
Cross-cancer Prediction: A Novel Machine Learning Approach to Discover Molecular Targets for Development of Treatments for Multiple Cancers.Cancer Inform. 2018 Oct 22;17:1176935118805398. doi: 10.1177/1176935118805398. eCollection 2018. Cancer Inform. 2018. PMID: 30364884 Free PMC article.
-
Triclosan induces apoptosis in Burkitt lymphoma-derived BJAB cells through caspase and JNK/MAPK pathways.Apoptosis. 2021 Feb;26(1-2):96-110. doi: 10.1007/s10495-020-01650-0. Epub 2021 Jan 2. Apoptosis. 2021. PMID: 33387145
-
Differential Roles for DUSP Family Members in Epithelial-to-Mesenchymal Transition and Cancer Stem Cell Regulation in Breast Cancer.PLoS One. 2016 Feb 9;11(2):e0148065. doi: 10.1371/journal.pone.0148065. eCollection 2016. PLoS One. 2016. PMID: 26859151 Free PMC article.
References
-
- Recht A, Come SE, Henderson IC, Gelman RS, Silver B, Hayes DF, et al. The sequencing of chemotherapy and radiation therapy after conservative surgery for early-stage breast cancer. N Engl J Med. 1996;334:1356–61. - PubMed
-
- Liang K, Lu Y, Jin W, Ang KK, Milas L, Fan Z. Sensitization of breast cancer cells to radiation by trastuzumab. Mol Cancer Ther. 2003;2:1113–20. - PubMed
-
- Debeb BG, Xu W, Woodward WA. Radiation resistance of breast cancer stem cells: understanding the clinical framework. J Mammary Gland Biol Neoplasia. 2009;14:11–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

