Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner
- PMID: 25375324
- PMCID: PMC4223067
- DOI: 10.1371/journal.ppat.1004502
Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner
Erratum in
-
Correction: Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner.PLoS Pathog. 2015 Feb 13;11(2):e1004709. doi: 10.1371/journal.ppat.1004709. eCollection 2015 Feb. PLoS Pathog. 2015. PMID: 25679792 Free PMC article.
Abstract
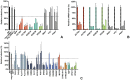
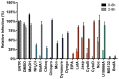
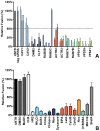
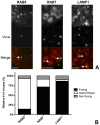
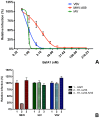
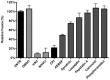
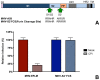
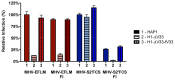
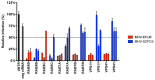
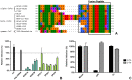
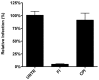
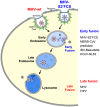
Enveloped viruses need to fuse with a host cell membrane in order to deliver their genome into the host cell. While some viruses fuse with the plasma membrane, many viruses are endocytosed prior to fusion. Specific cues in the endosomal microenvironment induce conformational changes in the viral fusion proteins leading to viral and host membrane fusion. In the present study we investigated the entry of coronaviruses (CoVs). Using siRNA gene silencing, we found that proteins known to be important for late endosomal maturation and endosome-lysosome fusion profoundly promote infection of cells with mouse hepatitis coronavirus (MHV). Using recombinant MHVs expressing reporter genes as well as a novel, replication-independent fusion assay we confirmed the importance of clathrin-mediated endocytosis and demonstrated that trafficking of MHV to lysosomes is required for fusion and productive entry to occur. Nevertheless, MHV was shown to be less sensitive to perturbation of endosomal pH than vesicular stomatitis virus and influenza A virus, which fuse in early and late endosomes, respectively. Our results indicate that entry of MHV depends on proteolytic processing of its fusion protein S by lysosomal proteases. Fusion of MHV was severely inhibited by a pan-lysosomal protease inhibitor, while trafficking of MHV to lysosomes and processing by lysosomal proteases was no longer required when a furin cleavage site was introduced in the S protein immediately upstream of the fusion peptide. Also entry of feline CoV was shown to depend on trafficking to lysosomes and processing by lysosomal proteases. In contrast, MERS-CoV, which contains a minimal furin cleavage site just upstream of the fusion peptide, was negatively affected by inhibition of furin, but not of lysosomal proteases. We conclude that a proteolytic cleavage site in the CoV S protein directly upstream of the fusion peptide is an essential determinant of the intracellular site of fusion.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Lysosomal Proteases Are a Determinant of Coronavirus Tropism.J Virol. 2018 Nov 27;92(24):e01504-18. doi: 10.1128/JVI.01504-18. Print 2018 Dec 15. J Virol. 2018. PMID: 30258004 Free PMC article.
-
Role of the spike glycoprotein of human Middle East respiratory syndrome coronavirus (MERS-CoV) in virus entry and syncytia formation.PLoS One. 2013 Oct 3;8(10):e76469. doi: 10.1371/journal.pone.0076469. eCollection 2013. PLoS One. 2013. PMID: 24098509 Free PMC article.
-
The Polybasic Cleavage Site in SARS-CoV-2 Spike Modulates Viral Sensitivity to Type I Interferon and IFITM2.J Virol. 2021 Apr 12;95(9):e02422-20. doi: 10.1128/JVI.02422-20. Print 2021 Apr 12. J Virol. 2021. PMID: 33563656 Free PMC article.
-
[Cell entry mechanisms of coronaviruses].Uirusu. 2009 Dec;59(2):215-22. doi: 10.2222/jsv.59.215. Uirusu. 2009. PMID: 20218330 Review. Japanese.
-
[Protease-dependent cell entry mechanism of coronaviruses].Uirusu. 2011 Jun;61(1):109-16. doi: 10.2222/jsv.61.109. Uirusu. 2011. PMID: 21972562 Review. Japanese.
Cited by
-
Rapid discovery and classification of inhibitors of coronavirus infection by pseudovirus screen and amplified luminescence proximity homogeneous assay.Antiviral Res. 2023 Jan;209:105473. doi: 10.1016/j.antiviral.2022.105473. Epub 2022 Nov 23. Antiviral Res. 2023. PMID: 36435212 Free PMC article.
-
TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells.Life Sci Alliance. 2020 Jul 23;3(9):e202000786. doi: 10.26508/lsa.202000786. Print 2020 Sep. Life Sci Alliance. 2020. PMID: 32703818 Free PMC article.
-
In silico anti-viral assessment of phytoconstituents in a traditional (Siddha Medicine) polyherbal formulation - Targeting Mpro and pan-coronavirus post-fusion Spike protein.J Tradit Complement Med. 2023 Jul 13;14(1):55-69. doi: 10.1016/j.jtcme.2023.07.004. eCollection 2024 Jan. J Tradit Complement Med. 2023. PMID: 38223813 Free PMC article.
-
The molecular virology of coronaviruses.J Biol Chem. 2020 Sep 11;295(37):12910-12934. doi: 10.1074/jbc.REV120.013930. Epub 2020 Jul 13. J Biol Chem. 2020. PMID: 32661197 Free PMC article. Review.
-
Characteristics of the Coronavirus Disease 2019 and related Therapeutic Options.Mol Ther Methods Clin Dev. 2020 Jun 24;18:367-375. doi: 10.1016/j.omtm.2020.06.013. eCollection 2020 Sep 11. Mol Ther Methods Clin Dev. 2020. PMID: 32665963 Free PMC article. Review.
References
-
- Okada Y (1969) Factors in fusion of cells by HVJ. Current topics in microbiology and immunology 48: 102–128. - PubMed
-
- Permanyer M, Ballana E, Este JA (2010) Endocytosis of HIV: anything goes. Trends in microbiology 18: 543–551. - PubMed
-
- Stein BS, Gowda SD, Lifson JD, Penhallow RC, Bensch KG, et al. (1987) pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell 49: 659–668. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

