Pharmacological inhibition of the chemokine CXCL16 diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury
- PMID: 25372401
- PMCID: PMC4221470
- DOI: 10.1371/journal.pone.0112327
Pharmacological inhibition of the chemokine CXCL16 diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury
Abstract
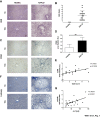
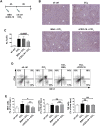
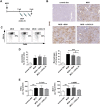
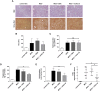
Non-alcoholic fatty liver disease (NAFLD) is a major cause of morbidity and mortality in developed countries, resulting in steatohepatitis (NASH), fibrosis and eventually cirrhosis. Modulating inflammatory mediators such as chemokines may represent a novel therapeutic strategy for NAFLD. We recently demonstrated that the chemokine receptor CXCR6 promotes hepatic NKT cell accumulation, thereby controlling inflammation in experimental NAFLD. In this study, we first investigated human biopsies (n = 20), confirming that accumulation of inflammatory cells such as macrophages is a hallmark of progressive NAFLD. Moreover, CXCR6 gene expression correlated with the inflammatory activity (ALT levels) in human NAFLD. We then tested the hypothesis that pharmacological inhibition of CXCL16 might hold therapeutic potential in NAFLD, using mouse models of acute carbon tetrachloride (CCl4)- and chronic methionine-choline-deficient (MCD) diet-induced hepatic injury. Neutralizing CXCL16 by i.p. injection of anti-CXCL16 antibody inhibited the early intrahepatic NKT cell accumulation upon acute toxic injury in vivo. Weekly therapeutic anti-CXCL16 administrations during the last 3 weeks of 6 weeks MCD diet significantly decreased the infiltration of inflammatory macrophages into the liver and intrahepatic levels of inflammatory cytokines like TNF or MCP-1. Importantly, anti-CXCL16 treatment significantly reduced fatty liver degeneration upon MCD diet, as assessed by hepatic triglyceride levels, histological steatosis scoring and quantification of lipid droplets. Moreover, injured hepatocytes up-regulated CXCL16 expression, indicating that scavenging functions of CXCL16 might be additionally involved in the pathogenesis of NAFLD. Targeting CXCL16 might therefore represent a promising novel therapeutic approach for liver inflammation and steatohepatitis.
Conflict of interest statement
Figures
Similar articles
-
Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury.Gut. 2012 Mar;61(3):416-26. doi: 10.1136/gutjnl-2011-300304. Epub 2011 Aug 3. Gut. 2012. PMID: 21813474
-
Chemokine receptor CXCR6-dependent hepatic NK T Cell accumulation promotes inflammation and liver fibrosis.J Immunol. 2013 May 15;190(10):5226-36. doi: 10.4049/jimmunol.1202909. Epub 2013 Apr 17. J Immunol. 2013. PMID: 23596313
-
CD44 is a key player in non-alcoholic steatohepatitis.J Hepatol. 2017 Aug;67(2):328-338. doi: 10.1016/j.jhep.2017.03.003. Epub 2017 Mar 16. J Hepatol. 2017. PMID: 28323124
-
Chemokines and Chemokine Receptors in the Development of NAFLD.Adv Exp Med Biol. 2018;1061:45-53. doi: 10.1007/978-981-10-8684-7_4. Adv Exp Med Biol. 2018. PMID: 29956205 Review.
-
Pathogenesis of NASH: How Metabolic Complications of Overnutrition Favour Lipotoxicity and Pro-Inflammatory Fatty Liver Disease.Adv Exp Med Biol. 2018;1061:19-44. doi: 10.1007/978-981-10-8684-7_3. Adv Exp Med Biol. 2018. PMID: 29956204 Review.
Cited by
-
Protective effect of lyophilized sapodilla (Manilkara zapota) fruit extract against CCl4-induced liver damage in rats.Saudi J Biol Sci. 2020 Sep;27(9):2373-2379. doi: 10.1016/j.sjbs.2020.05.010. Epub 2020 May 11. Saudi J Biol Sci. 2020. PMID: 32884419 Free PMC article.
-
Utility of Human Relevant Preclinical Animal Models in Navigating NAFLD to MAFLD Paradigm.Int J Mol Sci. 2022 Nov 25;23(23):14762. doi: 10.3390/ijms232314762. Int J Mol Sci. 2022. PMID: 36499091 Free PMC article. Review.
-
Fibrogenic Pathways in Metabolic Dysfunction Associated Fatty Liver Disease (MAFLD).Int J Mol Sci. 2022 Jun 23;23(13):6996. doi: 10.3390/ijms23136996. Int J Mol Sci. 2022. PMID: 35805998 Free PMC article. Review.
-
Host Genetic Variant in CXCL16 May Be Associated With Hepatitis B Virus-Related Acute Liver Failure.Cell Mol Gastroenterol Hepatol. 2019;7(2):477-479.e4. doi: 10.1016/j.jcmgh.2018.09.018. Epub 2018 Oct 17. Cell Mol Gastroenterol Hepatol. 2019. PMID: 30827414 Free PMC article. No abstract available.
-
Short-term supplementation of celecoxib-shifted butyrate production on a simulated model of the gut microbial ecosystem and ameliorated in vitro inflammation.NPJ Biofilms Microbiomes. 2020 Feb 19;6(1):9. doi: 10.1038/s41522-020-0119-0. NPJ Biofilms Microbiomes. 2020. PMID: 32075981 Free PMC article.
References
-
- Schuppan D, Schattenberg JM (2013) Non-alcoholic steatohepatitis: pathogenesis and novel therapeutic approaches. J Gastroenterol Hepatol 28 Suppl 1: 68–76. - PubMed
-
- Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F (2013) The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol 58: 593–608. - PubMed
-
- Bhala N, Jouness RI, Bugianesi E (2013) Epidemiology and natural history of patients with NAFLD. Curr Pharm Des 19: 5169–5176. - PubMed
-
- Jepsen P, Vilstrup H, Mellemkjaer L, Thulstrup AM, Olsen JH, et al. (2003) Prognosis of patients with a diagnosis of fatty liver–a registry-based cohort study. Hepatogastroenterology 50: 2101–2104. - PubMed
-
- Agopian VG, Kaldas FM, Hong JC, Whittaker M, Holt C, et al. (2012) Liver transplantation for nonalcoholic steatohepatitis: the new epidemic. Ann Surg 256: 624–633. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

